ART Guidelines
Table of Contents
How to use these guidelines
Abbreviations
MODULES
1. What’s new the 2023 guidelines update?
2. Nucleoside/nucleotide reverse transcriptase inhibitor class of antiretroviral drugs
3. Integrase strand transfer inhibitor class of antiretroviral drugs
4. Non-nucleoside reverse transcriptase inhibitor class of antiretroviral drugs
5. Protease inhibitor class of antiretroviral drugs
6. Initiation and timing of antiretroviral therapy
7. Baseline investigations
8. Viral load
9. CD4+ cell count
10. Resistance and genotyping
11. Initial antiretroviral therapy regimens for the previously untreated patient
12. Management of patients currently receiving first-line therapy
13. Management of patients starting or currently receiving second-line therapy
14. Third-line antiretroviral therapy
15. Laboratory monitoring of the efficacy and safety of antiretroviral therapy
16. Patients who return after stopping antiretroviral therapy
17. Drug-drug interactions
18. Tuberculosis
19. Pregnancy and breastfeeding
20. Liver disease
21. Renal disease
22. Psychiatric disease
23. Malaria
24. Antiretroviral drug-induced liver injury
25. Dyslipidaemia
26. Immune reconstitution inflammatory syndrome
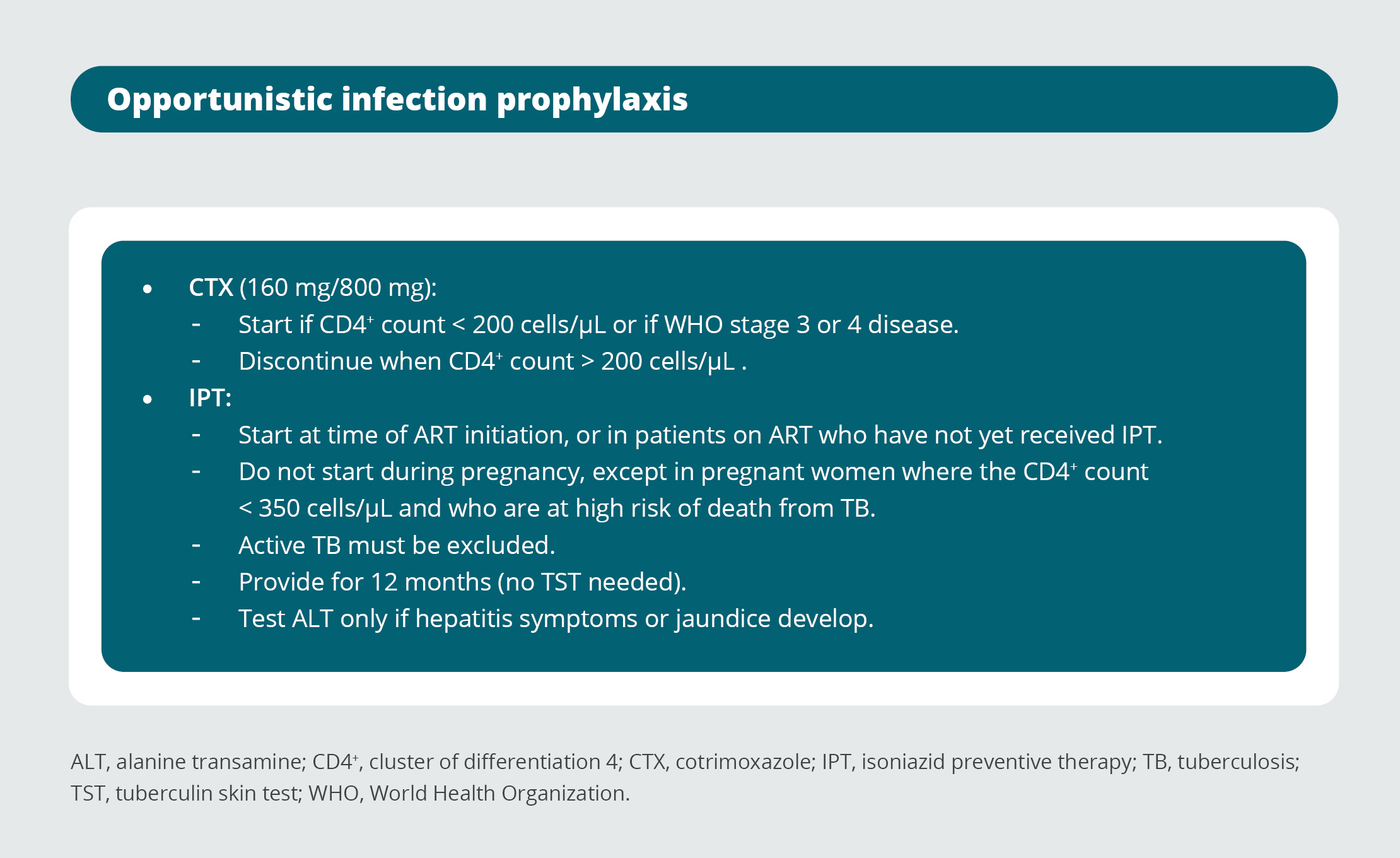
27. Opportunistic infection prophylaxis
28. Adherence
References
References

Useful algorithms
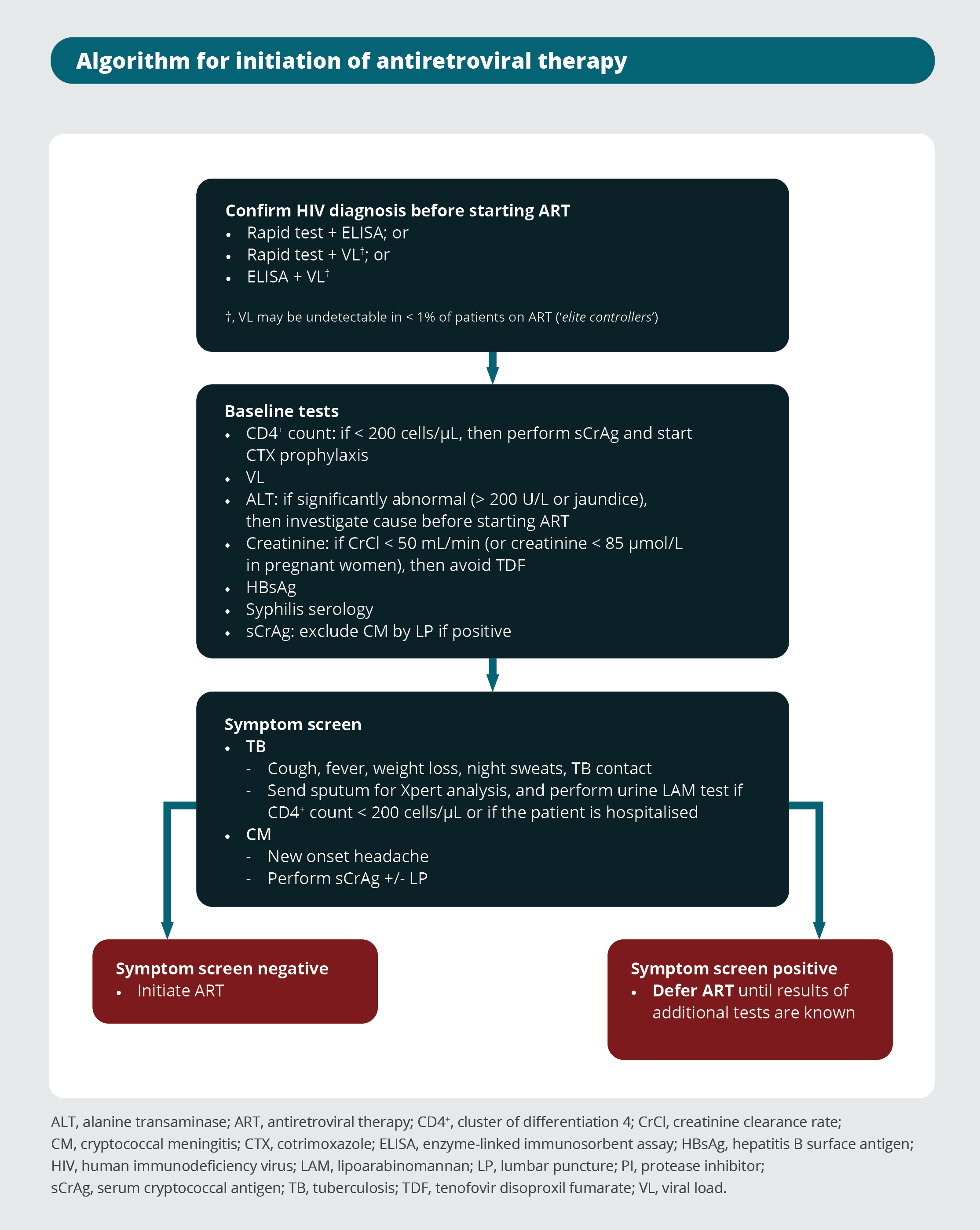
Algorithm for initiation of antiretroviral therapy
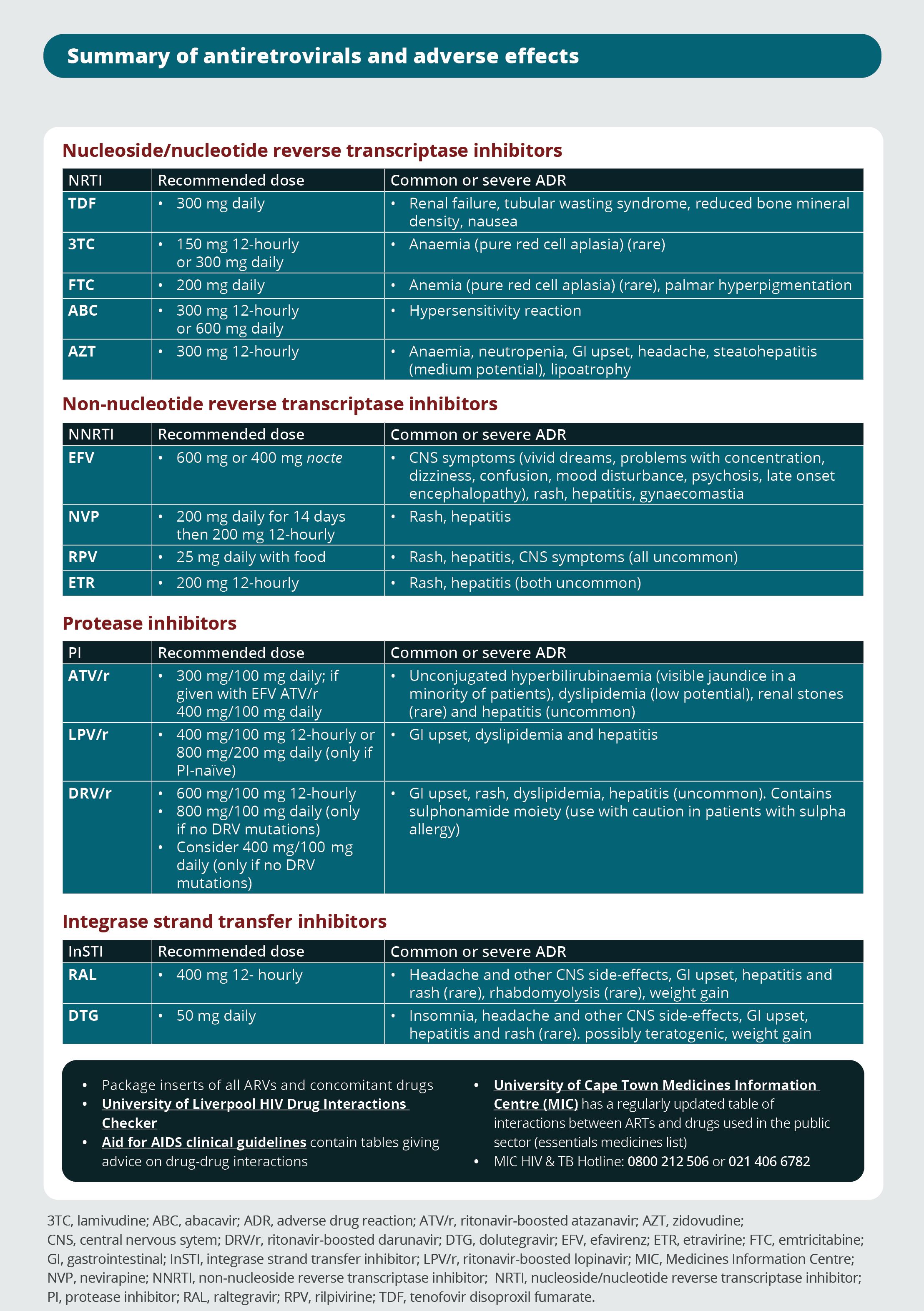
Summary of antiretrovirals and adverse effects
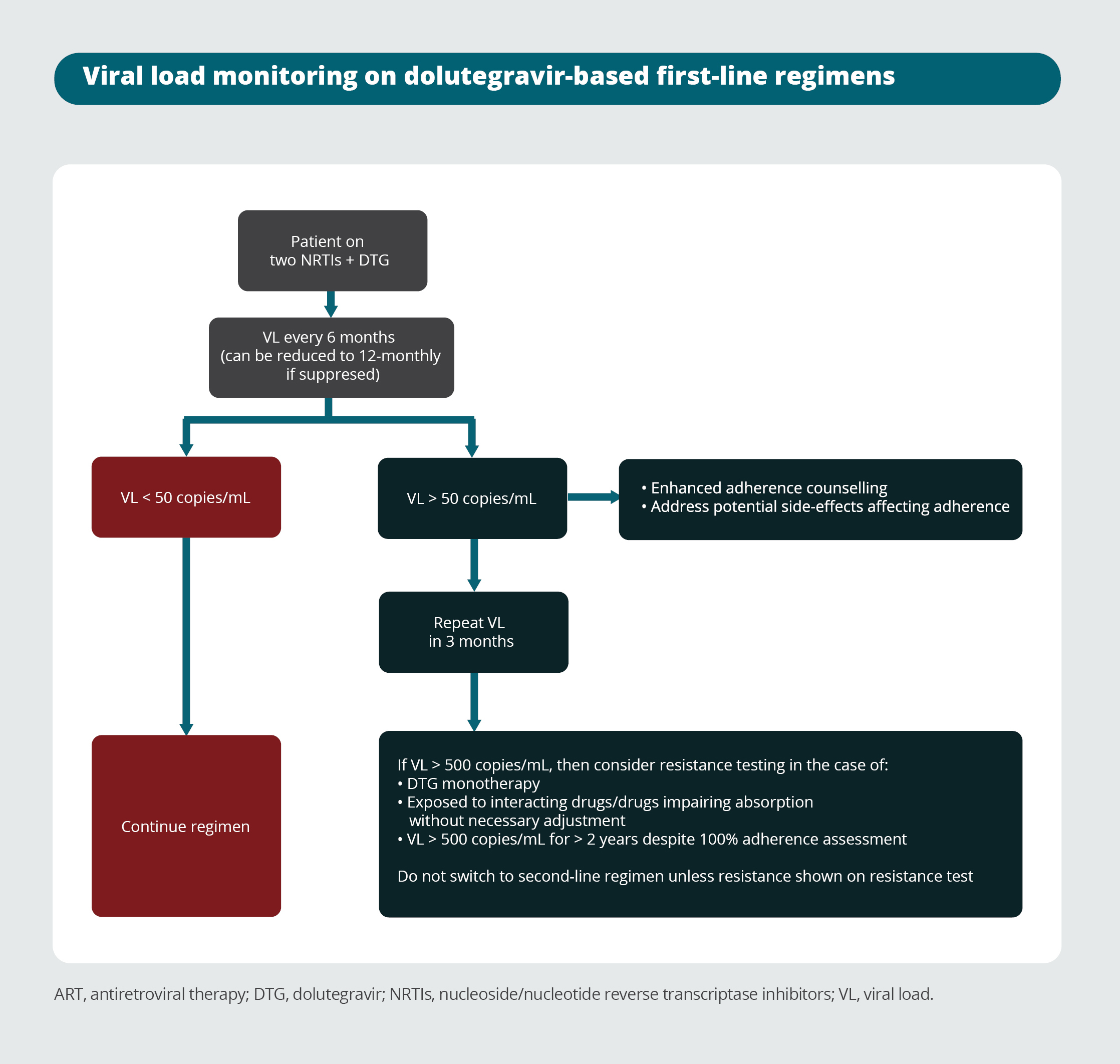
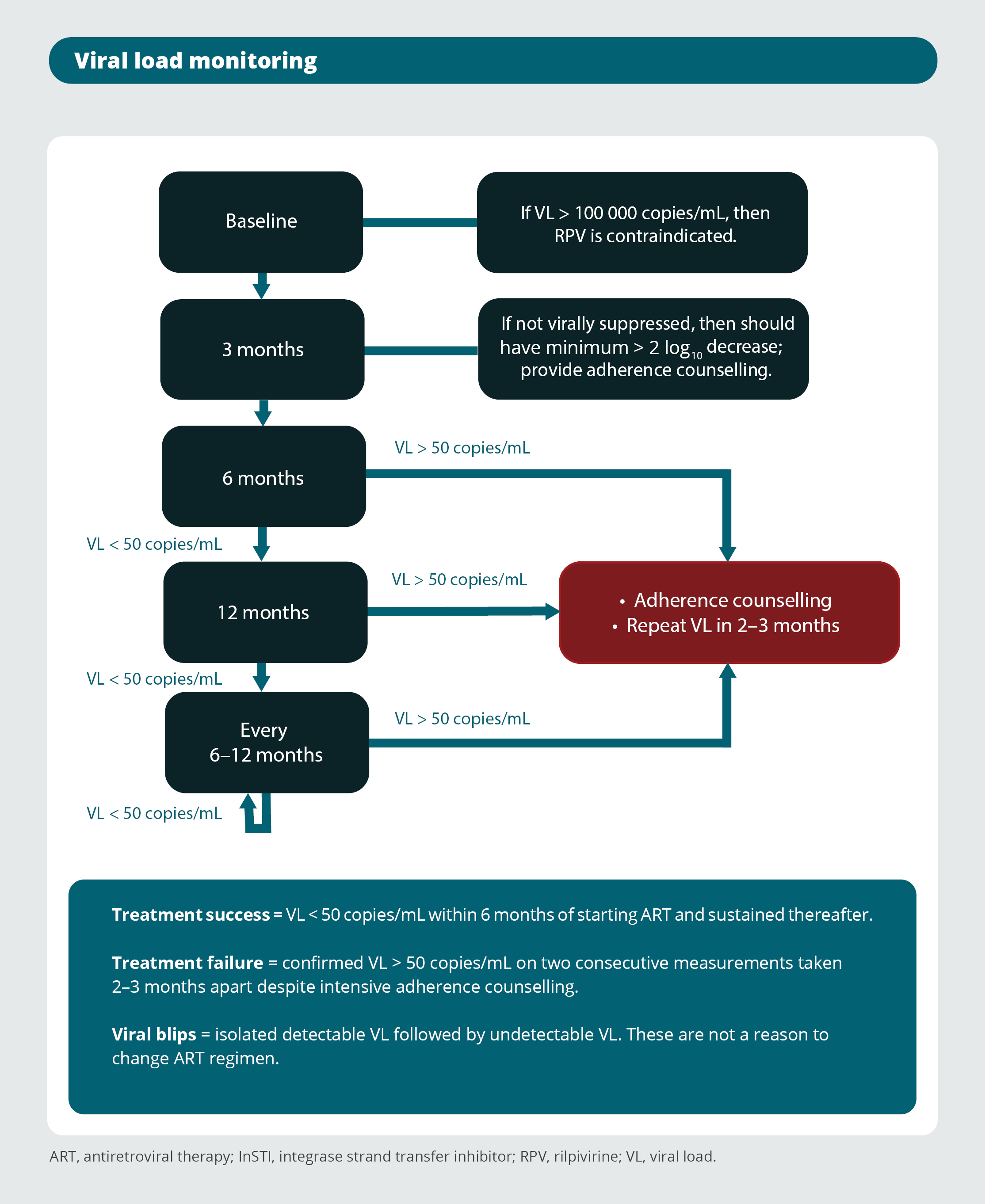
Viral load monitoring
CD4+ count monitoring

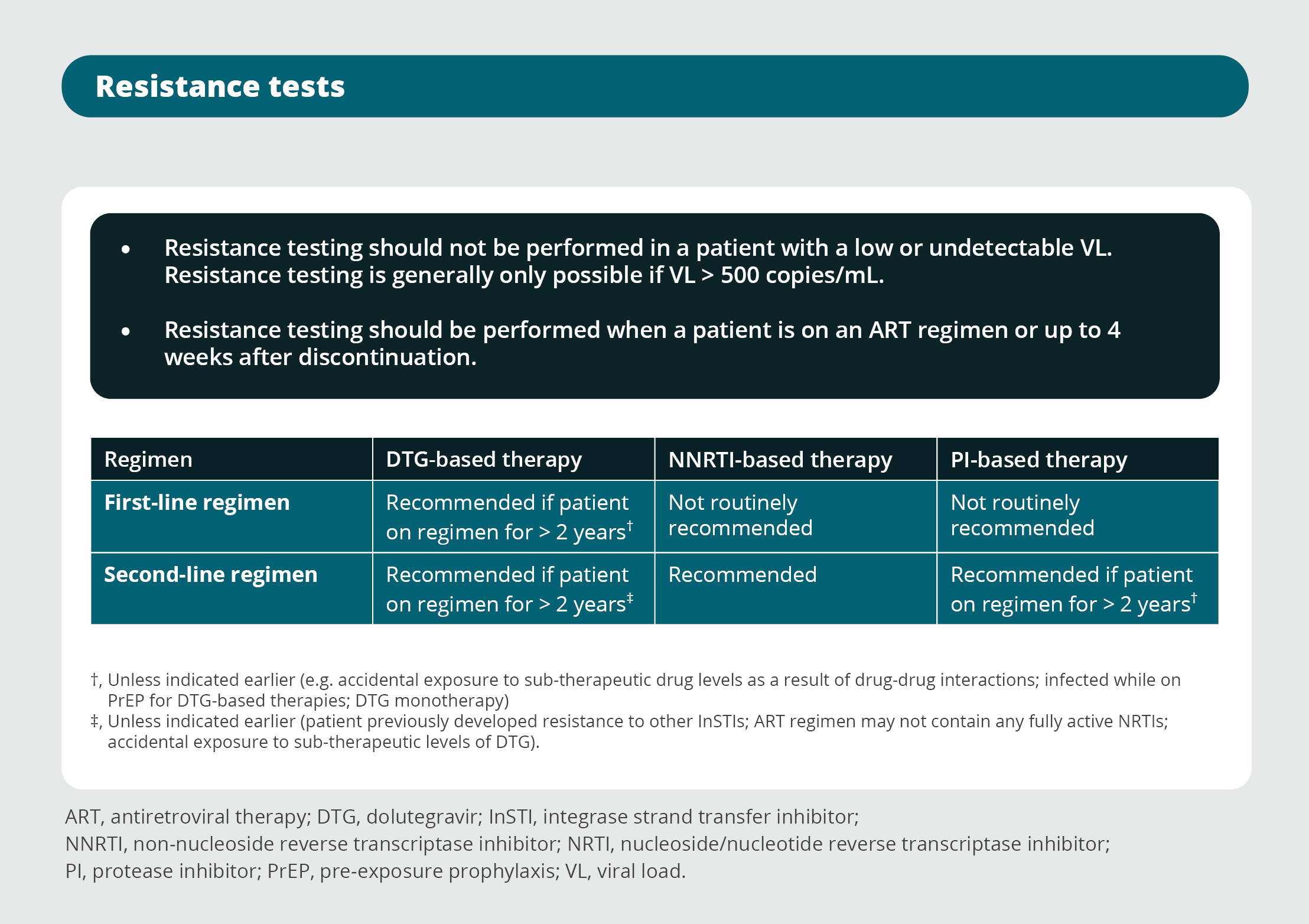
Resistance tests
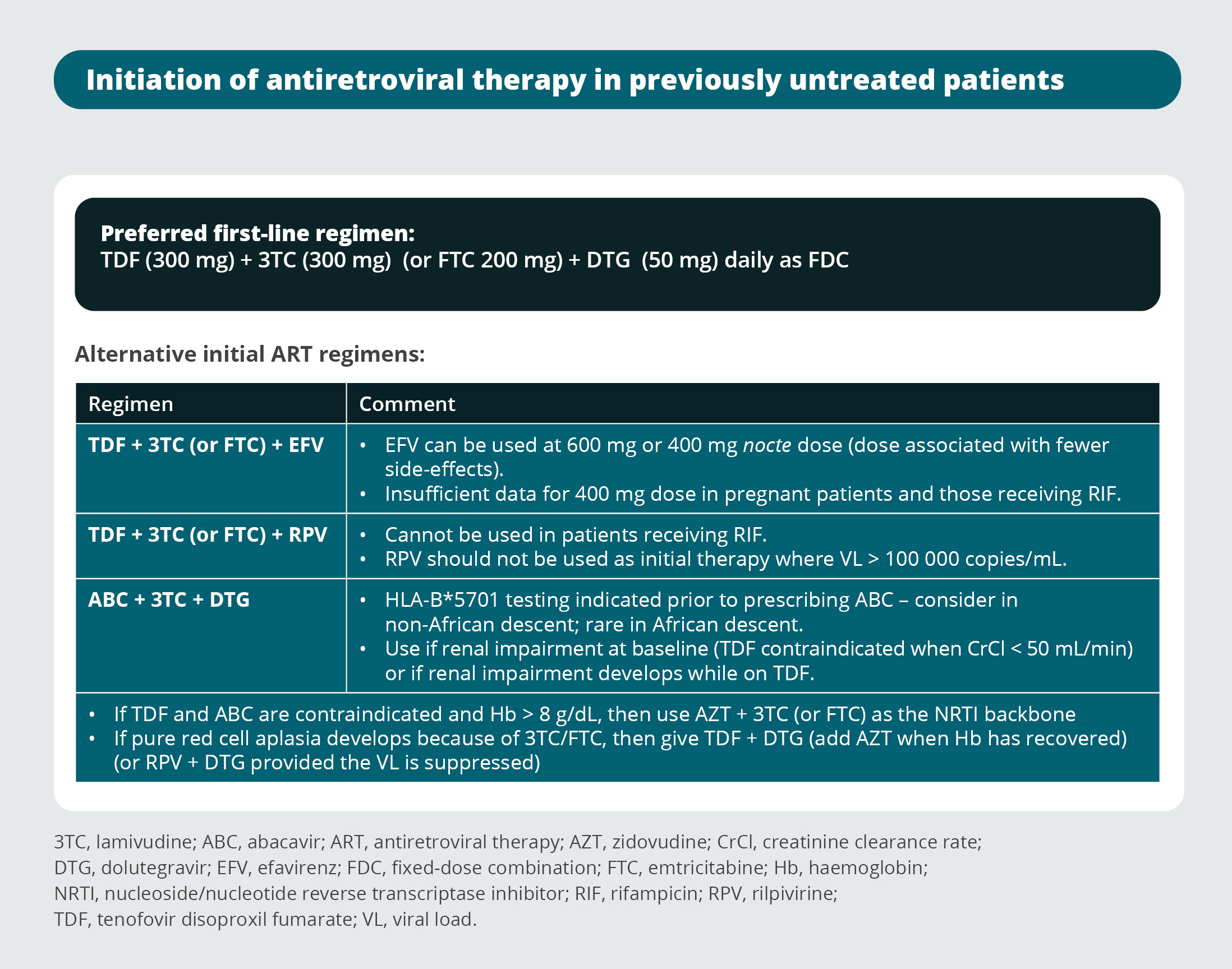
Initiation of antiretroviral therapy in previously untreated patients
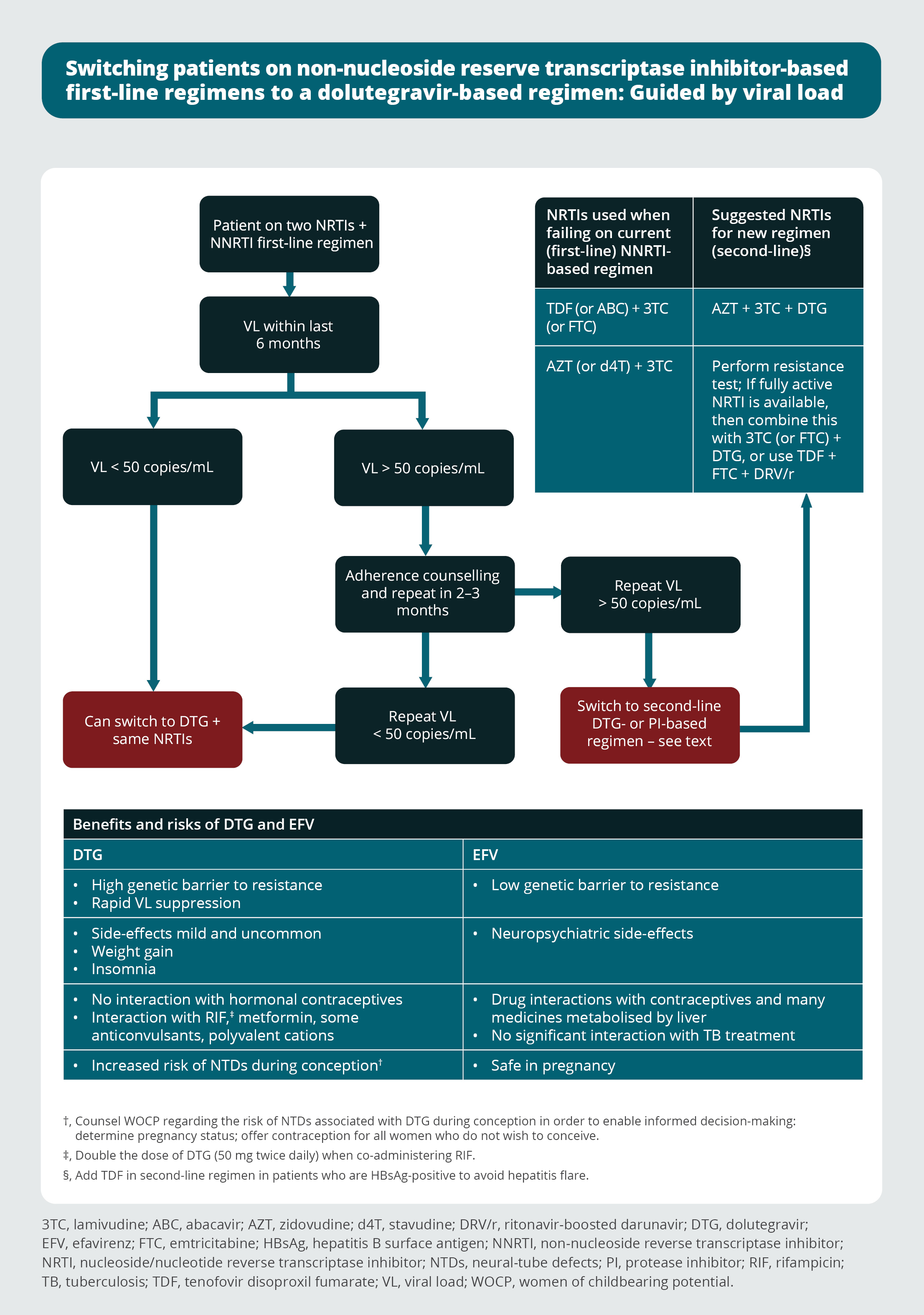
Switching patients on non-nucleoside reverse transcriptase inhibitor-based first-line regimens to a dolutegravir-based regimen: Guided by viral load
Protease inhibitor-based second-line regimens
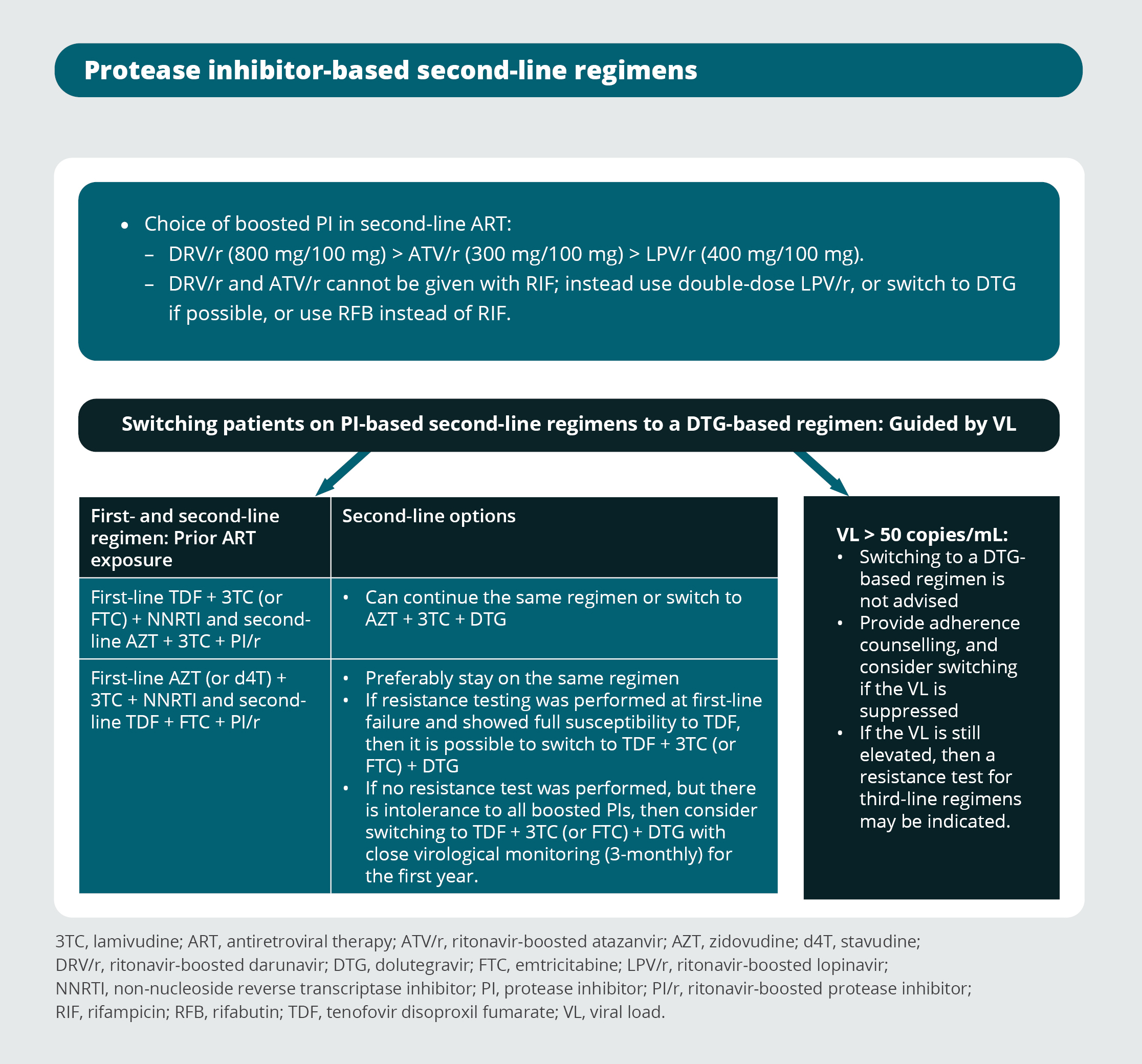
Third-line antiretroviral therapy regimens
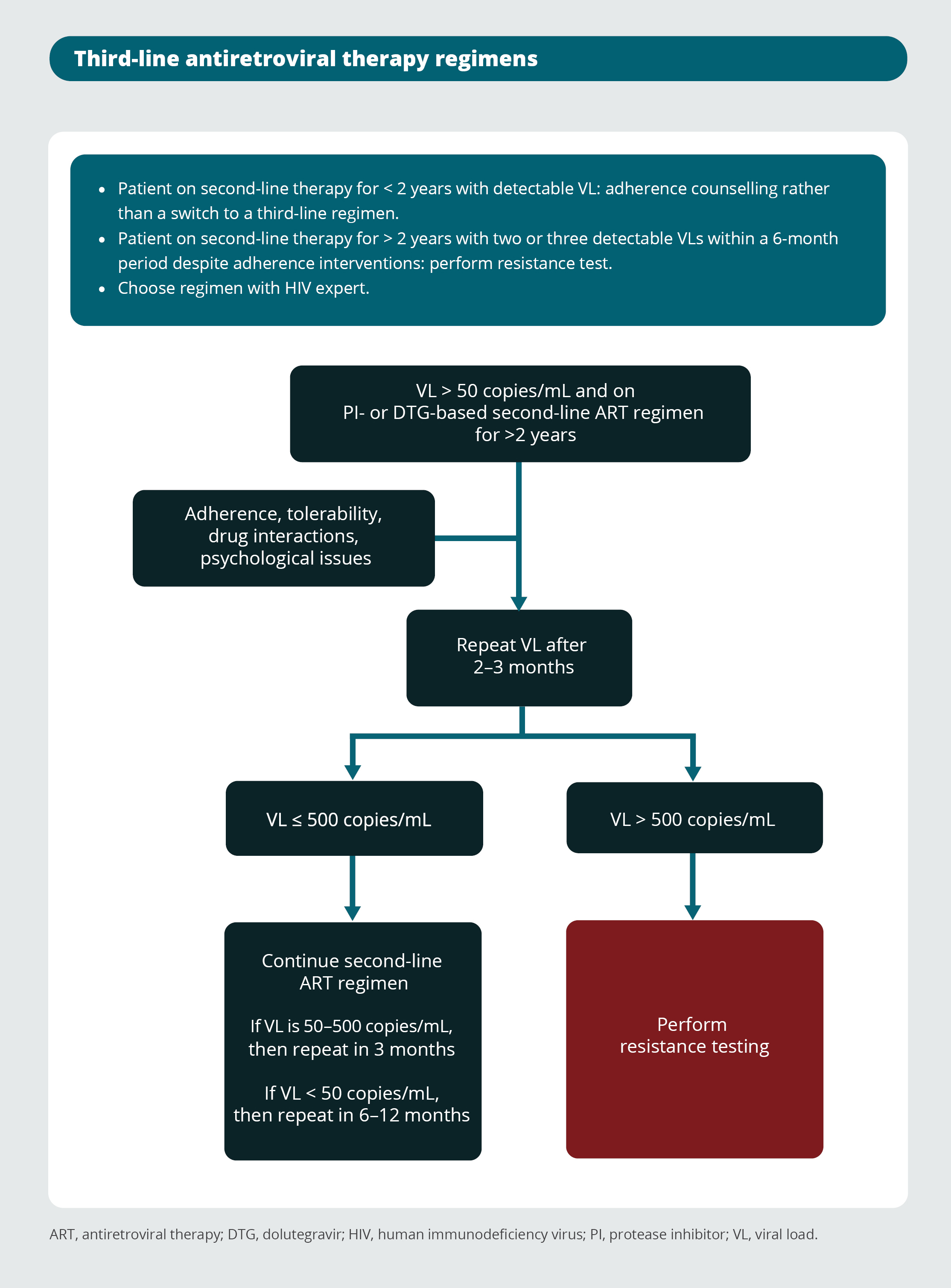
Monitoring of antiretroviral therapy
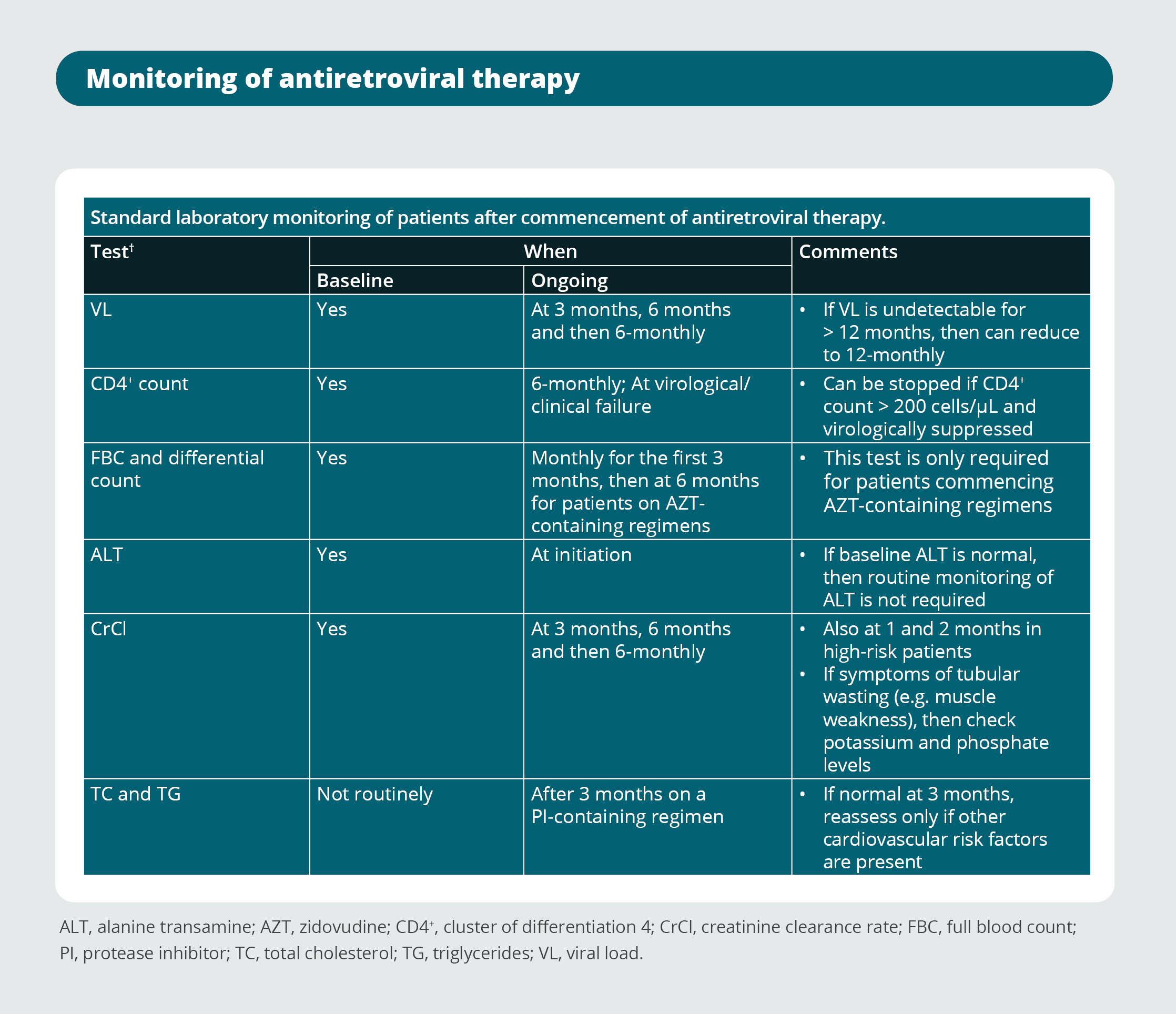
Opportunistic infection prophylaxis