ART Guidelines
References

 Key points
Key points - For a patient with a detectable VL on second-line therapy for < 2 years, intensified adherence counselling and support are required rather than a switch to a third-line therapy.
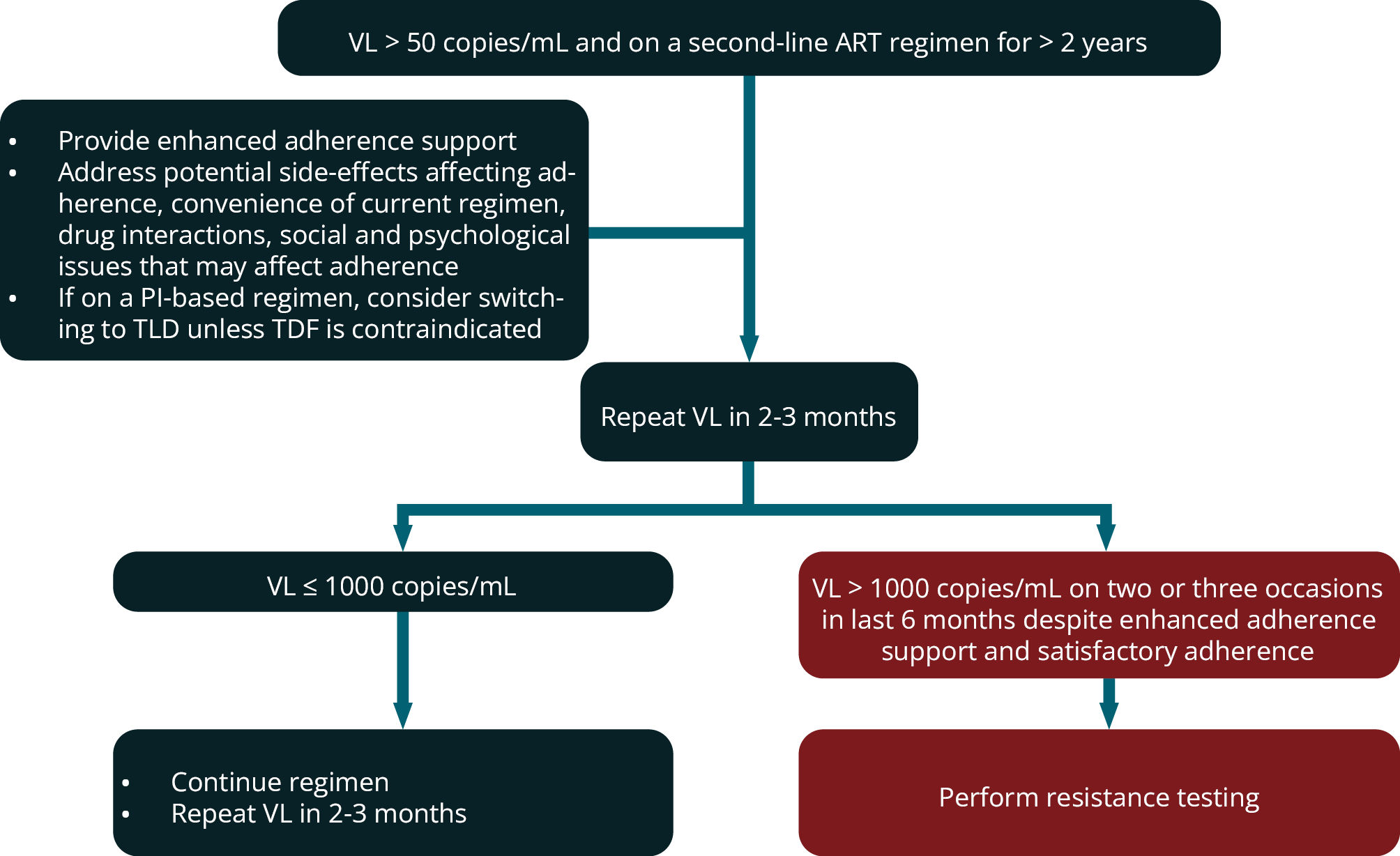
- For a patient on a second-line regimen for > 2 years with 2 or 3 VL measurements > 1000 copies/mL within a 6-month period despite adherence interventions that have been assessed to be satisfactory, then a resistance test should be performed.
- The choice of the third-line regimen should be made in conjunction with an HIV expert, based on treatment history and resistance test(s) result.
n a patient who has been on a second-line regimen for ≥ 2 years, if there are two or three VL measurements > 1000 copies/mL in a 6-month period despite adherence interventions, and adherence is assessed to be satisfactory (e.g. 100% pharmacy claims over 6 months), then a resistance test should be performed. If a patient who has been on secondline therapy for < 2 years is found to have a detectable VL, then a resistance test should not be performed; rather, the regimen should be reviewed with a view to making it more tolerable and convenient to facilitate adherence, and intensified adherence counselling and support provided. For a patient on a PI regimen, this will usually involve switching them to TLD, unless TDF is contraindicated (see module 13).
It is unlikely that significant resistance to a PI or DTG will have developed within 2 years. The exceptions include: a patient who in error was not prescribed LPV/r double dosing with concurrent RIF use who subsequently demonstrates a detectable VL, and a patient who has been taking an incorrectly low dose of medication. Such patients are eligible for resistance testing even if they have been on second-line therapy for < 2 years.
There should be documented PI or DTG resistance before switching to a third-line regimen. Resistance tests should be interpreted by an expert in conjunction with a full ART history. In many patients failing second-line regimens, there are no PI (or DTG) mutations found on resistance testing. In these patients, improved adherence is required rather than switching to a third-line regimen. Such patients should be switched to a simple and tolerable regimen to facilitate better adherence, which will usually be TLD unless TDF is contra-indicated in a patient currently on a PI regimen.
Figure 5 outlines indications for performing resistance testing in patients on second-line ART regimens.
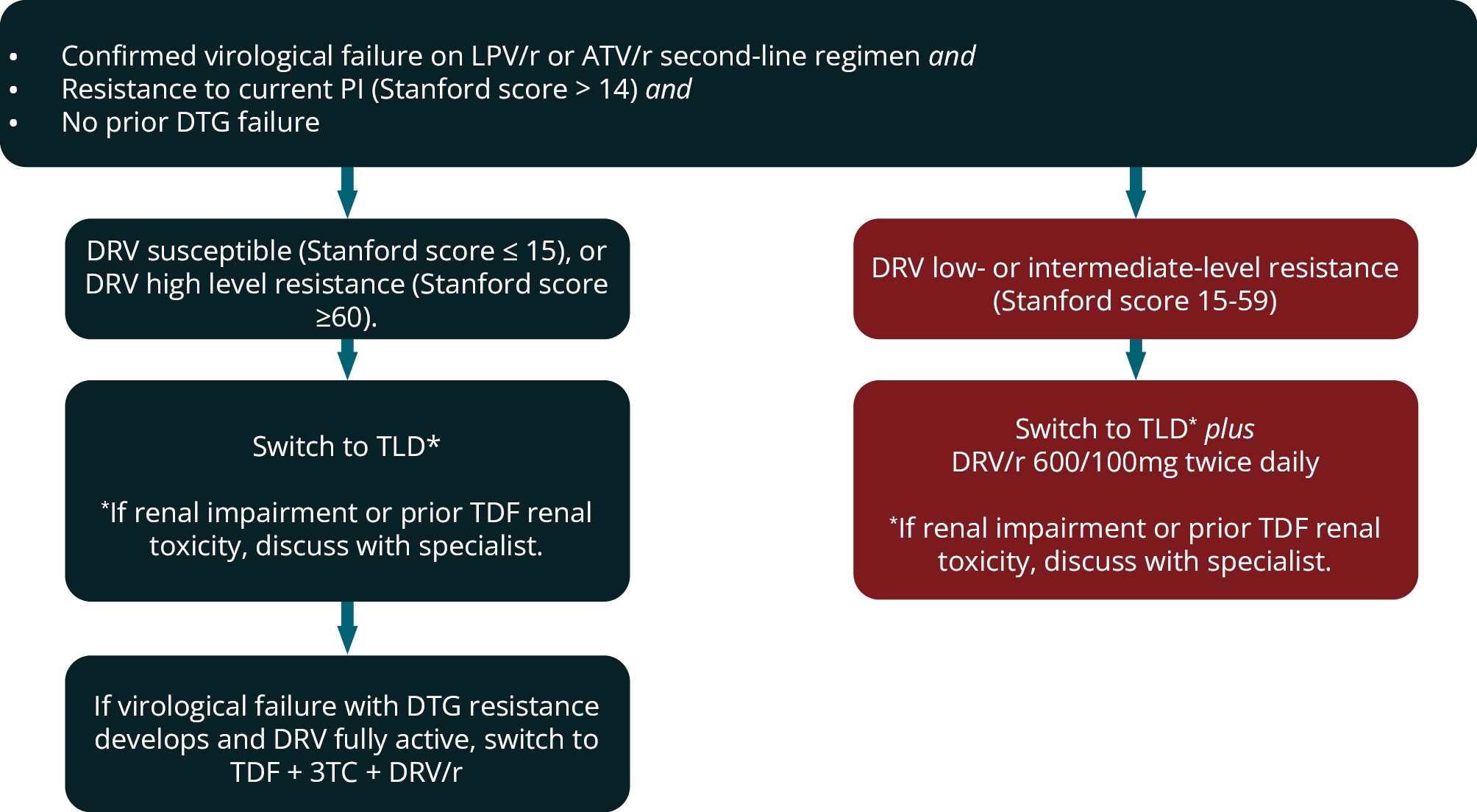
In most patients who experience virological failure on a second-line LPV/r or ATV/r regimen with low-, intermediate- or high-level resistance to the PI (i.e. Stanford Score > 14) a switch to TLD is recommended provided DRV is reported as fully susceptible and there has been no prior DTG failure. TLD will be an effective regimen in most patients even if TDF + 3TC resistance is present, based on evidence from the NADIA, VISEND and ARTIST trials. 83, 98 100 For the minority of patients who will go on to fail this third-line TLD regimen and develop DTG resistance then TDF + 3TC + DRV/r would be an appropriate subsequent regimen provided DRV is still fully active.
We therefore recommend avoiding switching to TLD alone after second-line ATV/r or LPV/r regimen failure if there is DRV cross-resistance reported on the resistance test. If DRV is reported as low- or intermediately susceptible on this resistance test (i.e. Stanford score 15-59), we rather recommend TLD plus DRV/r 600/100mg twice daily to provide a more robust regimen, because there is no fully active regimen available if the third-line regimen fails (Figure 6). If patients in this category cannot take TDF (renal impairment or prior TDF nephrotoxicity) the regimen should be discussed with an HIV expert.
FIGURE 5: Indications for performing resistance (genotype) testing in second-line antiretroviral therapy.
(ART, antiretroviral therapy; DTG, dolutegravir; LPV/r, ritonavir-boosted lopinavir; PI, protease inhibitor, VL, viral load.)
The usual option for patients who fail a DTG secondline regimen and are found to have DTG resistance is TDF + 3TC + DRV/r. However, if they are found to have PI resistance or are strongly suspected to have archived PI resistance, they may require additional drugs and treatment should be discussed with an HIV expert. Patients with renal impairment or prior TDF nephrotoxicity will also need an alternative regimen, and this should be discussed with an HIV expert
In patients failing a second-line DTG regimen who have not failed a PI-regimen previously, it can be assumed that DRV/r is fully active and can be used at 800 mg/100 mg daily. Based on the results of the NADIA trial, 83 it can be concluded that a TDF + 3TC + DRV/r regimen will be an active regimen with a high barrier to PI resistance even if there is resistance to the two NRTIs (TDF and 3TC).
Certain patients with long or complicated treatment histories may require additional drugs in their regimen and should be discussed with an HIV expert. These may include:
- RPV or ETV (provided Stanford score for the drug is < 30). Because most patients are not receiving a NNRTI at the time of failing second-line therapy, when a genotype resistance test is typically performed, prior NNRTI mutations related to first-line NNRTI failure may be archived at this time. Therefore, it is difficult to be certain from the resistance test performed at second-line failure, whether ETR/RPV are still active; however, data from South Africa suggest that the majority of patients who have failed NVP or EFV do not have high levels of resistance to ETR/RPV. 103
-
FIGURE 6: Indications for performing resistance (genotype) testing in second-line antiretroviral therapy. ART, antiretroviral therapy; DTG, dolutegravir; LPV/r, ritonavir-boosted lopinavir; PI, protease inhibitor, VL, viral load
- MVC (a CCR5 blocker) is a consideration in thirdline therapy; however, it is extremely costly and can only be used after a tropism test demonstrates that the patient’s circulating virus has sole tropism for the CCR5 co-receptor. MVC should only be considered when there is intermediate- or highlevel resistance to all PIs, all NNRTIs and all NRTIs, and there is not full susceptibility to DTG.
- RAL may still be active in patients with DTG resistance depending on the specific resistance mutations in the integrase region. Its use should be guided by resistance test report.
Decisions regarding third-line therapy need to be individualised in consultation with an HIV expert, taking into account treatment history (which drugs and classes the patient previously failed) and previous and current resistance test results.
Given the new evidence regarding the efficacy of TLD with compromised NRTIs, it may be possible to simplify the regimens of certain patients currently on multi-drug third-line regimens to improve adherence. This should only be done after a careful review of treatment history and all genotype results with an HIV expert. If the regimen is simplified to TLD, it must be ensured that a follow-on regimen is still available should that regimen fail and DTG resistance develop. This would typically require DRV to be fully susceptible. Simplification of DRV 600/100mg 12-hourly to 800/100mg once daily to reduce pill burden can also be considered if prior resistance tests demonstrated a Stanford score for DRV of 0.
Specific adherence counselling should be provided for patients preparing to start third-line ART, with a frank discussion that this regimen may be their last option for the foreseeable future
- Patients with a DRV score of 0 on Stanford Score (and no DRV mutations, see module 5) can take DRV/r 800 mg/100 mg once daily.
- Patients with prior virological failure on RAL and/or with a DTG score > 0 on integrase resistance testing should receive DTG 50 mg twice daily.
- We generally advise the continuation of NRTIs in the third-line regimen, even if there is documented NRTI resistance. 3TC (or FTC) resistance with the M184V mutation impairs viral replication, as does TDF resistance with the K65R mutation.
- In the SAILING trial, in treatment-experienced patients, a DTG regimen proved superior to RAL and fewer patients in the DTG arm developed treatment-emergent InSTI resistance. 104 Consequently, we no longer recommend use of RAL in third-line therapy unless DTG is not tolerated or based on resistance test result. We also recommend switching patients currently using RAL in third-line therapy to DTG, due to its higher barrier to resistance. 104 If such patients have a suppressed VL, then they can be switched to standard dose DTG 50 mg daily, but if they are not virologically suppressed, then we recommend a resistance test with a request for integrase sequencing, before switching. If there are InSTI mutations present that are associated with partially reduced susceptibility to DTG, then the DTG dose should be 50 mg twice daily if DTG is used.
- If viral suppression is not achieved on third-line therapy, there is still benefit in continuing failing ART, because of the residual partial activity and ‘crippling’ effect of such ART. ‘Crippling’ describes mutant viruses often having a lower replicative capacity. Provided that the VL can be maintained at < 10 000 copies/mL, the CD4+ count will usually be maintained or even increase. 82