ART Guidelines
References
 |
SOUTHERN AFRICAN HIV CLINICIANS SOCIETY GUIDELINES FOR ANTIRETROVIRAL THERAPY IN ADULTS: 2023 UPDATE |
 |
 |
 |
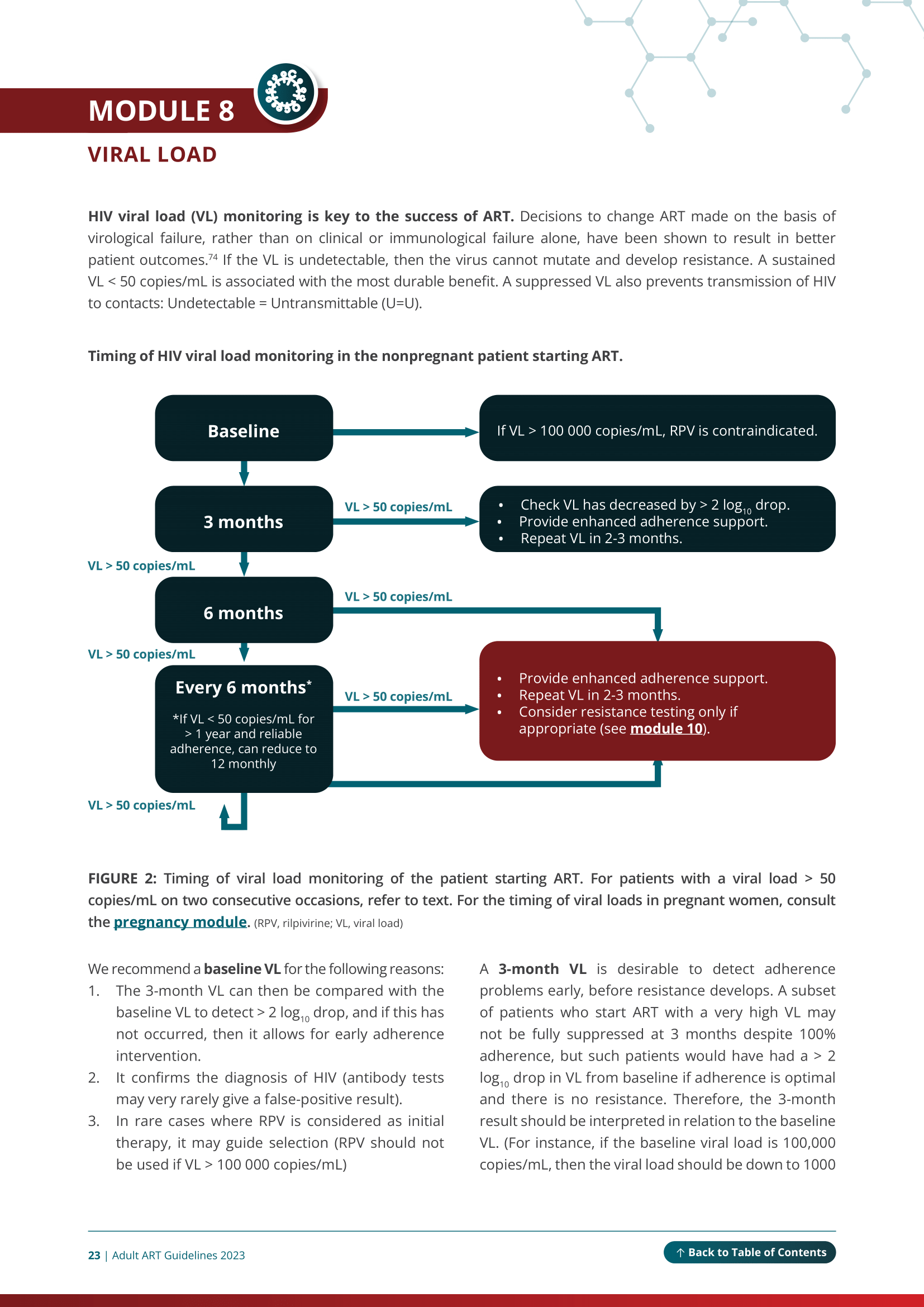 |
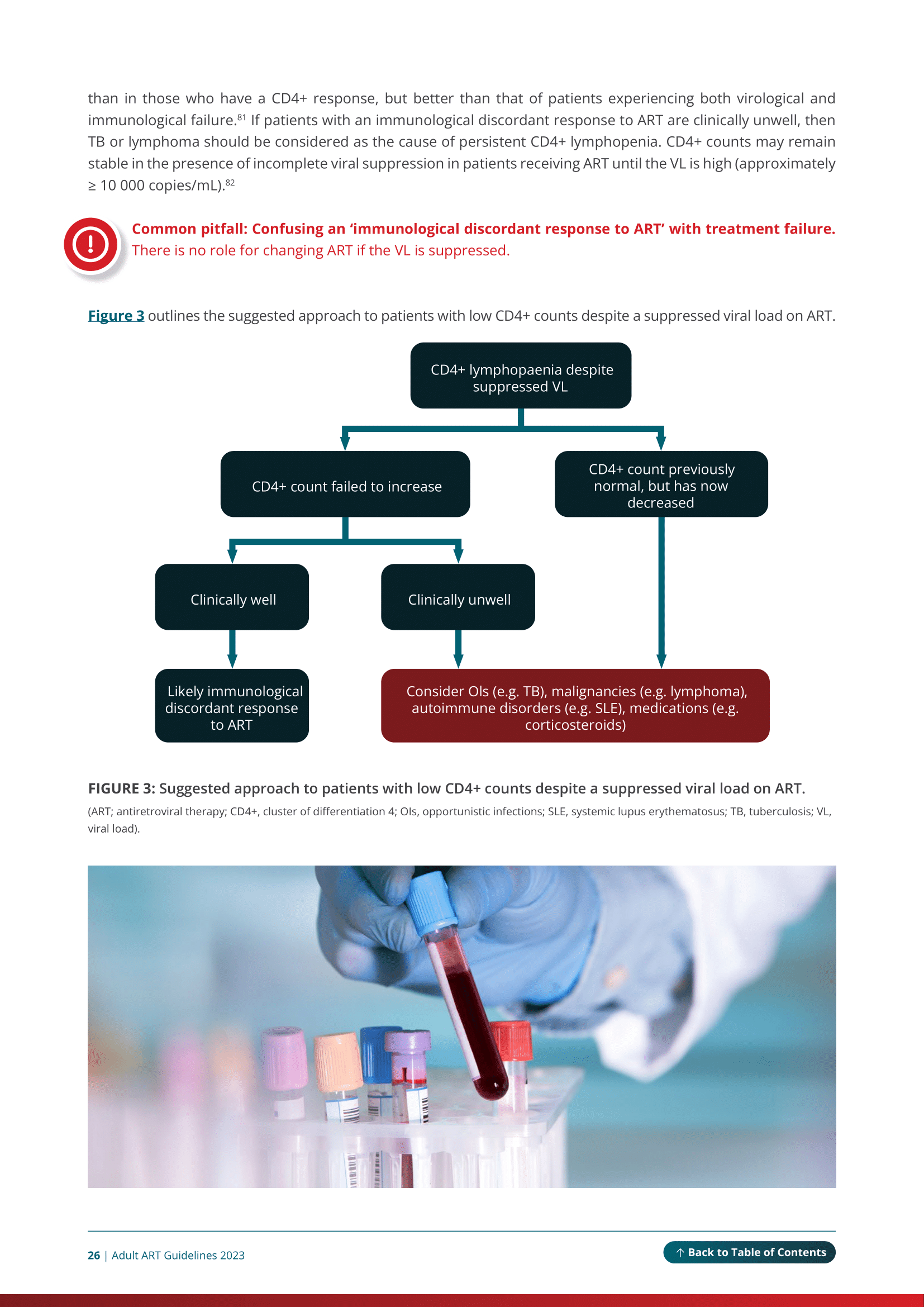
You can use the online version or download the PDF. When using the online version on a mobile device, or smaller screen, click on the top left-hand ‘Menu’ button to navigate to each module and access the abbreviations list. Every image can be enlarged and all references appear as numbered citations in the text. Tables in the guidelines are best viewed in landscape orientation when accessed via mobile device or tablet.
Additional guidance: Please regularly check into this page to get the latest information on these guidelines, obtain hints regarding what is in the pipeline, and access links to interesting articles.
Sign up for updates: Please be sure to sign up for updates to receive alerts regarding any updates relevant to these guidelines.
Feedback: Feel free send us feedback here
Authors: Jeremy Nel, Graeme Meintjes, Regina Osih (Chairpersons), Larisse Badenhorst, John Black, Rosie Burton, Nomathemba Chandiwana, Francesca Conradie, Natasha Davies, Mariam Edoo, Ute Feucht, Cloete Jansen Van Vuuren, John Joska, Richard Lessells, Gary Maartens, Pheto Mangena, Thandekile Manzini, Yunus Moosa, Ndiviwe Mphothulo, Jennifer Nash, Dulcy Rakumakoe, Evan Shoul, Phumla Sinxadi, David Spencer, Helen van der Plas, Camilla Wattrus, Jeannette Wessels, Joana Woods, Jarrod Zamparini (Authors in alphabetical order by surname)
Corresponding author: Jeremy Nel, [email protected]
Disclaimer: Specific recommendations provided here are intended only as a guide to clinical management, based on expert consensus and best current evidence. Treatment decisions for patients should be made by their responsible clinicians, with due consideration for individual circumstances. The most current version of this document should always be consulted.
