ART Guidelines
References

 Key points
Key points - DTG is the preferred integrase strand transfer inhibitor (InSTI) because it has a high barrier to resistance, is well tolerated, is available in a FDC formulation, and can be taken once daily.
- DTG causes a small increase in serum creatinine (usually <30 µmol /L) due to interference with tubular creatinine secretion; however, this does not represent a decline in renal function.
- Contrary to initial concerns, DTG does not cause neural tube defects, and should be freely offered to women of reproductive age, throughout pregnancy and breastfeeding.
- Although DTG is associated with more weight gain compared to other regimens, it appears to unmask it rather than cause it. There is no role for switching ART regimens in patients who experience significant weight gain on DTG.
Integrase strand transfer inhibitors (InSTIs) – often simply termed ‘integrase inhibitors’ – work by preventing the transfer of proviral DNA strands into the host chromosomal DNA. Currently, two InSTIs are available in Southern Africa: dolutegravir (DTG) and raltegravir (RAL). DTG is preferred due to its higher barrier to resistance, its availability in fixed-dose combination (FDC) formulation, and the ability to take the drug once daily. 17- 18
DTG use has been shown to be virologically superior to EFV-based ART in the SINGLE trial. 19 This difference was largely driven by the superior tolerability of the DTG arm: 2% vs. 10% in the EFV arm had an adverse event leading to discontinuation of the study drug. DTG showed superior rates of viral suppression (71% vs. 63% respectively at 144 weeks).
DTG-based regimens have also been shown to be superior to PI-based regimens when used as initial therapy. As first-line therapy, DTG was superior to DRV/r with respect to both viral suppression rates and side-effect profile in the FLAMINGO trial. 20 The ARIA trial of ART-naïve women demonstrated a superior viral suppression rate compared to ATV/r, and with fewer side-effects. 21 When compared to PIs in second line therapy, DTG is non-inferior to DRV/r but superior to LPV/r. 4, 22
A key advantage of DTG when used as initial therapy is its extremely high barrier to resistance, with treatmentemergent resistance being exceptionally rare. 23 This is less true of DTG when used in subsequent regimens following virological failure, where approximately 1-3% of patients can be expected to develop DTG resistance within 1-3 years. 4, 22, 24, 25 Prior InSTI exposure with RAL also puts a patient at an increased risk of DTG resistance.
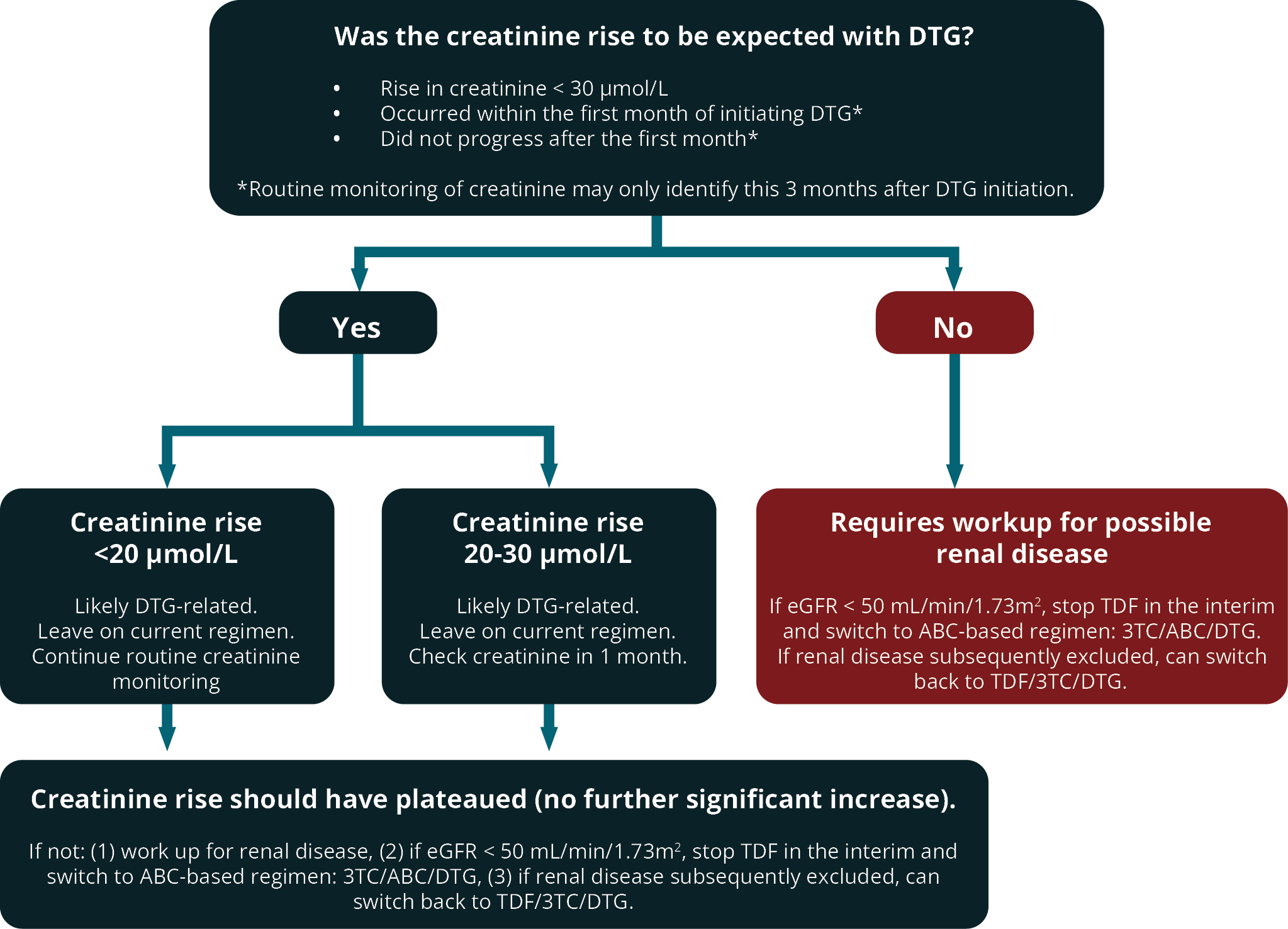
DTG and RAL are generally well tolerated, with most side-effects being mild and very rarely leading to discontinuation. DTG may cause a mild increase in serum creatinine due to interference with tubular secretion. This does not represent renal damage and is not an indication for switching to another drug. The rise in creatinine occurs within the first few weeks and then plateaus, and will persist for as long as the patient remains on DTG. Since DTG and TDF are commonly commenced together, it can be difficult to evaluate an early rise in serum creatinine. One suggested approach is outlined in Figure 1.
FIGURE 1: Assessment and management of rise in creatinine in patients on dolutegravir (DTG).
(DTG, dolutegravir; TDF, tenofovir; 3TC, lamivudine; ABC, abacavir; eGFR, estimated glomerular filtration rate)

Common pitfall: Assuming that the rise in creatinine seen in patients on DTG necessarily represents renal failure. In reality, the effect of DTG on creatinine secretion is of no consequence and does not represent a decline in renal function.
RAL and DTG can cause headaches when started, but this usually resolves. These drugs may also cause insomnia and neuropsychiatric side-effects. RAL and DTG can occasionally cause hypersensitivity rashes, including life-threatening rashes.
Although early results from the Tsepamo surveillance study in Botswana initially flagged a higher rate of neural tube defects (NTDs) among infants of women who were taking DTG at the time of conception compared to other ART, this has not been borne out by subsequent data from Tsepamo study, nor has it been seen in other similar cohorts from other countries. 26- 29 In addition, women on DTG-based regimens in pregnancy demonstrate faster time to viral suppression and better viral suppression rates at delivery, which may translate to better outcomes for both mother and child. 30- 33 Therefore, as for all adults, DTG is recommended as first-line treatment for pregnant and breastfeeding women, and for women of reproductive age.
Contrary to initial speculation that the integrase inhibitor class may cause weight gain, the association now appears not to be causal. Instead, the association may be the result of comparatively less metabolic toxicity than alternative older ART regimens (that impair weight gain through toxicity) combined with an initial return-to-health phenomenon, and an obesogenic environment. 34- 36 Given DTG-based ART regimens’ numerous advantages over comparators, DTG should not be withheld out of concern for weight gain. Clinicians should instead provide proactive advice for preventing weight gain through diet and exercise, as significant weight gain is almost universal after initiating antiretrovirals, and is progressive, especially among women. Evidence is accumulating for specific weight loss therapies such as glucagonlike peptide-1 (GLP-1) agonists, but these are likely to be unaffordable and currently out of reach of the vast majority of patients. There is no role for switching from DTG-containing regimens in patients gaining weight. See Box 1.

Common pitfall: Not starting DTG because of a fear of weight gain. Contrary to initial speculation, DTG does not appear to cause weight gain. Rather, physicians should alert patients to the possibility of weight gain when initiating any ART regimen, because of the return-to-health phenomenon and an obesogenic environment.
BOX 1: Advice on weight gain in patients on DTG-based regimens.
What the evidence suggests:
- Data from ADVANCE, NAMSAL and other high-quality trials show an association between dolutegravir and weight gain, particularly in women. In the ADVANCE cohort, median weight gain by 192 weeks was 5.8 kg for those on TDF/FTC/DTG compared to 3.3 kg for those on TDF/FTV/EFV.
- Most of the weight gain happens in the first year of starting DTG, and regardless of regimen, a component of the weight gain frequently represents a return-to-health phenomenon, whereby weight lost due to uncontrolled HIV is regained once HIV replication is suppressed.
- Subsequent analysis of the ADVANCE cohort revealed that the weight trajectory in patients on EFV had been determined largely by their genetics: those with a CYP2B6 genotype that enabled rapid metabolism of EFV had similar weight gain to participants in the DTG arm, intermediate EFV metabolisers gained almost no weight, and slow metabolisers (i.e., those with the highest serum EFV levels) lost weight.
- EFV is known to cause mitochondrial toxicity as well as inhibition of genes controlling adipogenesis and lipid accretion, reduced release of adipokines and increased pro-inflammatory catabolic cytokines. It may also mediate weight loss by neuropsychiatric adverse effects, by impairing appetite.
- Together, these results suggest that the difference in weight gain seen between EFV and DTG in ADVANCE and similar trials is likely to be due to genetically-predisposed EFV-related toxicities impairing weight gain rather than DTG-mediated weight gain.
Key management principles:
- Clinicians initiating or switching patient to DTG should take care not to unnecessarily imply that DTG “causes” weight gain, as this may lead to suboptimal treatment adherence in patients who do subsequently gain significant weight.
- The possibility of weight gain should be discussed by clinicians when starting all ART regimens, as obesity is an increasing problem in HIV care, particularly but not exclusively amongst women.
- Proactive advice for preventing weight gain through diet and exercise should be given to all patients starting or switching ART. Evidence is accumulating for specific weight loss therapies such as GLP-1 agonists, but these are likely to be unaffordable and out of reach of the vast majority of patients currently.
- Since the failure to gain weight in EFV-containing regimens is frequently mediated by toxic levels of EFV because of genetically slower drug metabolism, there is no current role for switching from DTG-containing regimens in patients gaining weight. In addition, drugs such as EFV are associated with inferior viral suppression rates compared to DTG.
Dosage and common adverse drug reactions of InSTIs are described in Table 3.
| TABLE 3: Dosage and common adverse drug reactions of integrase strand transfer inhibitors available in southern Africa. | ||
| Drug | Recommended dosage | Common or severe ADR |
|---|---|---|
| DTG | 50 mg daily | Insomnia, headache and other CNS side-effects, gastrointestinal upset, hepatitis and rash (rare) can be accompanied by severe systemic hypersensitivity reaction |
| RAL | 400 mg 12-hourly | Headache and other CNS side-effects, gastrointestinal upset, hepatitis and rash (rare), rhabdomyolysis (rare) |
|
CNS, central nervous system; DTG, dolutegravir; RAL, raltegravir.
†Life-threatening reactions are indicated in bold. |
||
Key drug-drug interactions involving DTG are summarised in Table 4.
In general, patients on rifampicin (RIF)-based tuberculosis regimens should receive DTG 12-hourly until 2 weeks after stopping RIF, as RIF decreases DTG drug levels. However, a large observational cohort study from Botswana suggested that HIV viral suppression rates were not statistically significantly different between those who received DTG 12-hourly and those who received it daily. 37 This was recently confirmed by a randomised placebo-controlled trial done in Cape Town (RADIANT-TB trial), where viral suppression rates at week 24 were 83% for both those given DTG 12-hourly and those who received the drug daily. 38 Thus, DTG may be given as a daily dose in patients on RIF who are on first-line DTG-based regimens. However, for those who have a prior history of treatment failure on a different regimen, in whom the companion NRTIs may not have full activity, there is still limited evidence on the optimal DTG dose. Pending further data, we suggest such patients continue to receive DTG 12-hourly.
| TABLE 4: Key drug-drug interactions with dolutegravir. | |
| Drug | Action required |
|---|---|
| Rifampicin (RIF) | Pending further data, for patients on DTG who have a history of virologic failure on a previous regimen, we suggest DTG be administered twice-daily (i.e. 50 mg 12-hourly) until 2 weeks after stopping RIF. For patients on DTG with no prior history of virological failure, no change in DTG dosing is required. |
| Metformin | Do not exceed metformin 500 mg 12-hourly. |
| Carbamazepine, phenytoin | Give alternative anticonvulsant (e.g. lamotrigine, topiramate or levetiracetam). If carbamazepine is used, then administer DTG 12-hourly. |
| Polyvalent cation-containing agents (e.g. antacids, laxatives, sucralfate, and iron, calcium and zinc supplements). | For magnesium-/aluminium-containing antacids, administer > 2 hours after or > 6 hours before DTG dose. For iron/calcium supplements, either take with food, otherwise apply intervals above. For pregnancy supplements, also apply timing intervals above. |
| Etravirine | Do not use DTG + etravirine together unless a boosted PI is also used in the combination. |
|
DTG, dolutegravir; RIF, rifampicin, PI, protease inhibitor. |
|