ART Guidelines
References

HIV viral load (VL) monitoring is key to the success of ART. Decisions to change ART made on the basis of virological failure, rather than on clinical or immunological failure alone, have been shown to result in better patient outcomes. 74 If the VL is undetectable, then the virus cannot mutate and develop resistance. A sustained VL < 50 copies/mL is associated with the most durable benefit. A suppressed VL also prevents transmission of HIV to contacts: Undetectable = Untransmittable (U=U).
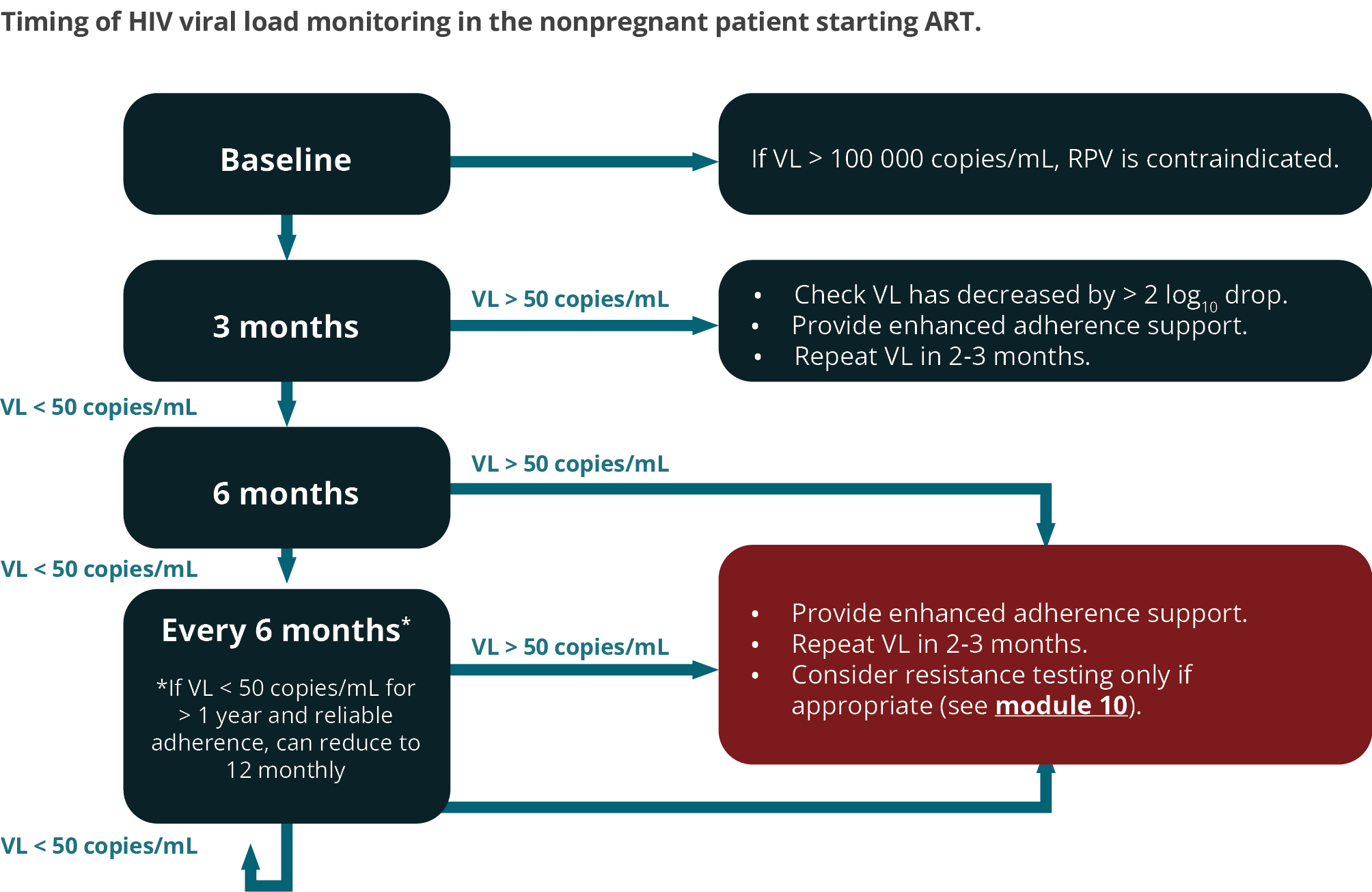
FIGURE 2: Timing of viral load monitoring of the patient starting ART. For patients with a viral load > 50 copies/mL on two consecutive occasions, refer to text. For the timing of viral loads in pregnant women, consult the pregnancy module. (RPV, rilpivirine; VL, viral load)
We recommend performing a baseline VL assessment for the following reasons:
- The 3-month VL can then be compared with the baseline VL to detect > 2 log10 drop, and if this has not occurred, then it allows for early adherence intervention
- It confirms the diagnosis of HIV (antibody tests may very rarely give a false-positive result).
- In rare cases where RPV is considered as initial therapy, it may guide selection (RPV should not be used if VL > 100 000 copies/mL)
A 3-month VL is desirable to detect adherence problems early, before resistance develops. A subset of patients who start ART with a very high VL may not be fully suppressed at 3 months despite 100% adherence, but such patients would have had a > 2 log10 drop in VL from baseline if adherence is optimal and there is no resistance. Therefore, the 3-month result should be interpreted in relation to the baseline VL. (For instance, if the baseline viral load is 100,000 copies/mL, then the viral load should be down to 1000 copies/mL or less at 3 months). All patients who have a detectable VL at 3 months should receive additional adherence interventions. In general, a patient’s VL declines fastest on InSTI-based regimens.
If the 3-month VL is undetectable, then VL monitoring is recommended at 6 months and every 6 months thereafter. In patients who have an undetectable VL for > 12 months, and who demonstrate reliable adherence and follow-up, it may be acceptable to reduce the frequency of VL monitoring to 12-monthly.
If the VL is > 50 copies/mL at any stage, then this should be an indication for urgent action. The patient should receive counselling and interventions should be implemented to improve adherence. A repeat measurement of VL should then be done in 2–3 months.
Treatment success is defined as a decline in VL to < 50 copies/mL within 6 months of commencing ART, and sustained thereafter. A VL > 50 copies/mL is robustly associated with subsequent virological failure. 75, 76 Sustained viral replication, even at these low levels, can lead to the accumulation of resistance mutations (although this has not yet been definitively established in the case of dolutegravir).
Treatment failure is defined as a confirmed VL > 1000 copies/mL on two consecutive measurements taken 2–3 months apart. The implication of this depends on the regimen the patient is on. Patients on DTG-based therapy as their initial ART regimen are extremely unlikely to have developed resistance at the point of treatment failure, but this is not necessarily true of other regimens, or of DTG-based regimens when there has been a history of failure with a prior ART regimen.
Isolated detectable HIV VLs < 1000 copies/mL, followed by an undetectable VL, are termed ‘viral blips’ and alone are not a reason to change the ART regimen.
- Viral blips can be caused by immune activation (such as from an acute infection), variability in the laboratory testing thresholds, or intermittent poor adherence. Provided they are infrequent, and the viral load returns to being undetectable at the next measurement, they are not regarded as consequential.
A high VL can be attributed to one or more of these three factors:
- Inadequate patient adherence (most commonly). This may sometimes be out of the patient’s control (e.g. stock-outs, unplanned facility closure). See Table 28.
- Resistance to the prescribed ART – including both acquired and transmitted drug resistance.
- Inadequate ART drug levels as a result of altered pharmacokinetics, such as absorption difficulties, or drug-drug interactions.
These explanations are not mutually exclusive. For instance, inadequate patient adherence may lead to the development of drug resistance.
Transmitted drug resistance is only likely to be a concern for the rare patients who initiate therapy on an NNRTI, as by far the commonest transmitted mutation is K103N, which has little effect on viral fitness and can therefore persist in the population even in the absence of drug pressure. 77 Transmitted drug resistance to other drug classes is unusual and often of minimal clinical importance; therefore firstline therapy with a DTG-based regimen is unlikely to be affected by this phenomenon.

