ART Guidelines
References

 Key points
Key points - Patients currently on TDF + FTC/3TC + EFV or NVP regimens should be switched to TLD regardless of whether their VL is suppressed or not . Ensure that those that are virally unsuppressed receive enhanced adherence support.
- The typical criteria of two VL measurements greater than a certain threshold despite an adherence intervention to define virological failure and direct a switch to second-line are not appropriate for DTGbased first-line regimens. Rather, in patients started on a first-line DTG regimen, we recommend switching to second-line therapy only if there is demonstrated DTG resistance.
In contrast to previous guidelines, we now advise that patients currently on TDF +FTC/3TC and EFV or NVP be switched to TLD regardless of whether their VL is suppressed or not suppressed. There are multiple lines of evidence supporting this recommendation:
- In the SINGLE trial, DTG was superior to EFV in first-line ART. 94
- DTG has a higher barrier to resistance than NNRTIs which improves the likelihood of sustained virologic suppression. 94 In an observational study among patients who were virologically suppressed those who did not switch to TLD from non-DTG regimens were significantly more likely to lose virological suppression. 95
- In patients who have an unsuppressed VL and have developed NRTI mutations, there is evidence that a DTG-based regimen is virologically superior to a LPV/r based regimen, 96 and that TLD is equivalent to a DRV/r- 83, 97 or an ATV/r-based 98 regimen.
- Maintaining TDF in second-line is superior to switching to AZT, 83, 97 hence we no longer recommend an NRTI switch from TDF to AZT in patients switching to second-line from a failing TDF + FTC/3TC + NNRTI, but rather using TLD.
A subset of patients switched from EFV to DTG may experience weight gain. This is experienced in patients who are slow metabolisers of EFV and therefore have higher drug exposures while on EFV. The weight gain is not directly caused by DTG but is rather due to the removal of the effect of EFV causing weight loss in the context of a lifestyle that promotes weight gain. Patients should be informed about this phenomenon. We do not recommend switching back to EFV, but rather a focus on diet and exercise interventions (see module 3).
An additional benefit of switching from NVP to DTG is switching from a twice-daily to once-daily regimen, potentially facilitating improved adherence.
In patients currently on an ABC + 3TC + EFV/NVP due to renal impairment or previous TDF nephrotoxicity or patients found to have renal impairment (eGFR < 50 mL/min/1.73m2) when the switch is being considered, then TDF cannot be used, and the options are:
If the VL is suppressed:
- TAF + FTC + DTG (if eGFR 30 – 50 mL/min/1.73m2). If available, TAF may be used as a standalone drug as part of a regimen if eGFR is > mL/min/1.73m2)
- ABC + 3TC + DTG
If the VL is not suppressed:
- TAF + FTC + DTG (if eGFR 30 – 50 mL/min/1.73m2)
- ABC + 3TC + DTG
- ABC + 3TC + DRV/r
In patients who develop acute kidney injury while on TDF and are switched to ABC, but where TDF was not the cause of the injury, when the eGFR has normalised, it is important to a switch back to the original TDF-based regimen rather than remaining on an ABC-based regimen.
It is important to note that the trials demonstrating the virological efficacy of a DTG or boosted PI regimen with compromised NRTIs have not included regimens with ABC + 3TC as the NRTI combination and therefore there is no direct evidence to support the virological efficacy of ABC + 3TC with DTG or PIs when both ABC and 3TC are compromised by resistance mutations. ABC has a different resistance mutation profile compared to TDF, and therefore caution needs to be taken in extending the findings of the EARNEST, SECOND LINE, SELECT, NADIA, VISEND and ARTIST trials to ABC + 3TC secondline regimens. Given this lack of data and a possible risk that virological failure could be more frequent compared with TLD in second-line, patients should be closely monitored on second-line regimens of ABC + 3TC + PI or ABC + 3TC + DTG.
Clinicians should consider switching virologically suppressed patients on RPV + two NRTI regimens from RPV to DTG, because of DTG’s higher barrier to resistance, but there is no direct evidence comparing the virological efficacy of DTG versus RPV first-line regimens. Patients experiencing virologic failure on a RPV-based regimen should be switched to a DTGbased regimen (TLD if eGFR > 50 ml/min/1.73m2 ).
Another option in patients who are virologically suppressed (VL < 50 copies/mL) while receiving a regimen of NNRTI + two NRTIs, and who have never experienced virological failure, is a switch to the two-drug combination of DTG + RPV. Data from two clinical trials (SWORD I and II) 99 show that this regimen maintains virological suppression as a switch strategy in patients who have not previously experienced virological failure. This should not be done in patients who have chronic hepatitis B as TDF and 3TC (or FTC) should always form part of their treatment. Hepatitis B surface antigen status should therefore always be tested before making this switch.
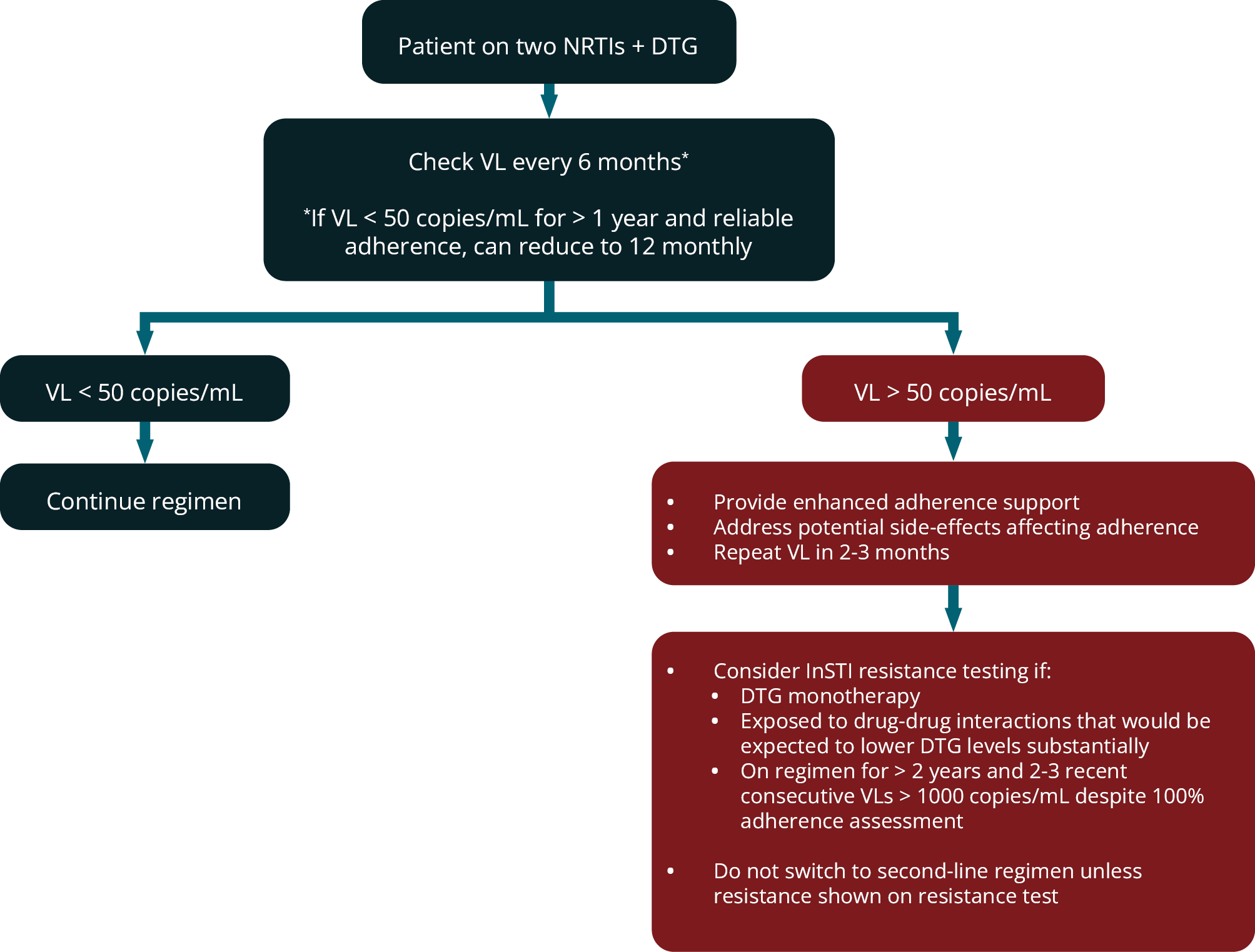
Such patients should have their VL measured 6–12-monthly (see modules 8 and 15). We have previously used the criteria of two VL measurements > 1000 copies/mL despite an adherence intervention to define virological failure and the need to switch from first- to second-line ART. This was appropriate for patients on NNRTI-based first-line regimens because of the low barrier to resistance of the NNRTI class. However, considerations are very different with DTGbased first-line regimens. In several clinical trials of DTG in first-line therapy, no DTG resistance has been described despite some patients having virological failure; and in clinical practice very few cases of DTG resistance have been described worldwide when the drug has been used as part of a three-drug first-line regimen. 89 Therefore, it would be inappropriate to use the same criteria for switching to second-line ART for DTG as it is likely that most patients with two unsuppressed VLs will not have resistance and rather require improved adherence on the same first-line regimen to achieve suppression. For that reason, we only recommend switching from first-line DTG-based ART to second-line if resistance testing demonstrates DTG resistance.
Until further data are available, in patients with an unsuppressed VL on DTG -based first-line ART, we recommend enhanced adherence counselling. The tolerance of the regimen should also be addressed as the regimen may need to be switched due to side-effects. Integrase resistance testing should be considered in these situations:
- Previous DTG monotherapy (DTG resistance has been more frequently described in this situation) 89
- Patient on DTG-based a regimen for > 2 years and 2-3 recent VLs consecutively > 1000 copies/mL (despite adherence interventions, 100% pharmacy refills and self-reported adherence)
- Prolonged period of exposure to a co-medication that has a drug-drug interaction that reduces exposure to DTG.
If a resistance test is performed, then this should include sequencing of the integrase region and should be limited to the integrase region if the laboratory makes this option available. The clinician should only switch from a DTG-based first-line regimen to second-line therapy if DTG resistance is detected . The choice of drugs in second-line are discussed in module 13.
These recommendations are based on accumulated information on the resistance barrier to DTG to date suggesting that DTG resistance is extremely rare when the drug is used in a three-drug first-line regimen. These recommendations may be updated when more data become available regarding incidence and risk factors for DTG resistance with more widespread use in routine clinical practice.
Figure 4 outlines the virological monitoring of patients on DTG-based first-line ART and the recommended response to results.
FIGURE 4: Virological monitoring of patients receiving DTG-based first-line ART and response to results. (DTG, dolutegravir; NRTIs, nucleoside reverse transcriptase inhibitors; VL; viral load)