Page 23 - Nursing Matters June 2021 Vol 12
P. 23
Was the mother on therapy during
pregnancy or breastfeeding?
What treatment did the mother take and for
how long?
Was child breastfed?
Did child receive any ARV at birth/ after
birth/ during breastfeeding? State ARV and
duration
ADHERENCE IN LAST 3 – 6 MONTHS
Regular clinic attendance
On-time pharmacy refill
Correct pill counts
Treatment partner observes taking of
medication
Alcohol / drug abuse
Severe GIT or other side effects experienced current issues
If adherence problem, what interventions were
undertaken to address the issue?
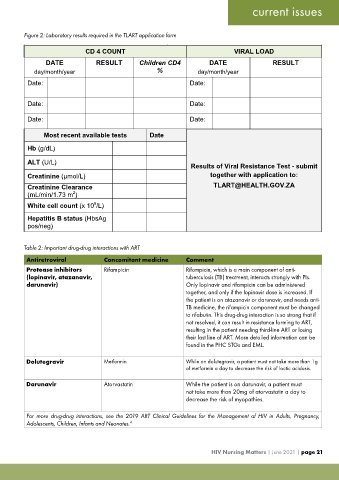
Figure 2: Laboratory results required in the TLART application form
CD 4 COUNT VIRAL LOAD
DATE RESULT Children CD4 DATE RESULT
day/month/year % day/month/year
Date: Date:
Date: Date:
Date: Date:
Most recent available tests Date
Hb (g/dL)
ALT (U/L)
Results of Viral Resistance Test - submit
Creatinine (µmol/L) together with application to:
Creatinine Clearance [email protected]
(mL/min/1.73 m )
2
9
White cell count (x 10 /L)
Hepatitis B status (HbsAg
pos/neg)
Table 2: Important drug-drug interactions with ART
Antiretroviral Concomitant medicine Comment
Protease inhibitors Rifampicin Rifampicin, which is a main component of anti-
(lopinavir, atazanavir, tuberculosis (TB) treatment, interacts strongly with PIs.
darunavir) Only lopinavir and rifampicin can be administered
together, and only if the lopinavir dose is increased. If
the patient is on atazanavir or darunavir, and needs anti-
TB medicine, the rifampicin component must be changed
to rifabutin. This drug-drug interaction is so strong that if
not resolved, it can result in resistance forming to ART,
resulting in the patient needing third-line ART or losing
their last line of ART. More detailed information can be
found in the PHC STGs and EML.
Dolutegravir Metformin While on dolutegravir, a patient must not take more than 1g
of metformin a day to decrease the risk of lactic acidosis.
Darunavir Atorvastatin While the patient is on darunavir, a patient must
not take more than 20mg of atorvastatin a day to
decrease the risk of myopathies.
For more drug-drug interactions, see the 2019 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy,
Adolescents, Children, Infants and Neonates. 4
HIV Nursing Matters | June 2021 | page 21