Page 369 - SAHCS HIVMed Journal Vol 20 No 1 2019
P. 369
Page 8 of 12 Original Research
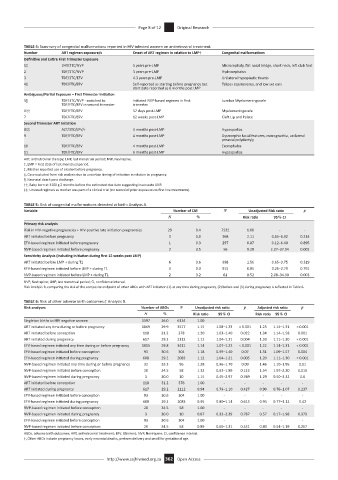
TABLE 4: Summary of congenital malformations reported in HIV-infected women on antiretroviral treatment.
Number ART regimen exposure/s Onset of ART regimen in relation to LMP† Congenital malformations
Definitive and Entire First Trimester Exposure
1‡ D4T/3TC/NVP 5 years pre-LMP Microcephaly, flat nasal bridge, short neck, left club foot
2 TDF/3TC/NVP 3 years pre-LMP Hydrocephalus
3 TDF/3TC/EFV 4.3 years pre-LMP Unilateral hypoplastic thumb
4§ TDF/FTC/EFV Self-reported as starting before pregnancy but Talipes equinovarus, and low set ears
start date recorded as 6 months post LMP
Ambiguous/Partial Exposure – First Trimester Initiation
5¶ TDF/3TC/NVP - switched to Initiated NVP-based regimen in first Lumbar Myelomeningocele
TDF/FTC/EFV in second trimester trimester
6†† TDF/FTC/EFV 57 days post-LMP Myelomeningocele
7 TDF/FTC/EFV 12 weeks post-LMP Cleft Lip and Palate
Second Trimester ART Initiation
8‡‡ AZT/3TC/LPV/r 4 months post-LMP Hypospadias
9 TDF/FTC/EFV 4 months post-LMP Dysmorphic facial features, micrognathia, unilateral
preaxial polydactyly
10 TDF/FTC/EFV 4 months post-LMP Exomphalos
11 TDF/FTC/EFV 6 months post-LMP Hypospadias
ART, antiretroviral therapy; LMP, last menstrual period; NVP, Nevirapine.
†, LMP = First date of last menstrual period.
‡, Mother reported use of alcohol before pregnancy.
§, Case excluded from risk analysis due to uncertain timing of initiation in relation to pregnancy.
¶, Neonatal death post discharge.
††, Baby born at 3100 g 2 months before the estimated due date suggesting inaccurate LMP.
‡‡, Unusual regimen as mother was part of a clinical trial (no record of prior exposures to first line treatments).
TABLE 5: Risk of congenital malformations detected at birth: Analysis A.
Variable Number of CM N Unadjusted Risk ratio p
N % Risk ratio 95% CI
Primary risk analysis
Risk in HIV-negative pregnancies + HIV-positive late initiation pregnancies 29 0.4 7532 1.00 - -
ART initiated before pregnancy 3 0.8 368 2.11 0.65–6.92 0.214
EFV-based regimen initiated before pregnancy 1 0.3 297 0.87 0.12–6.40 0.895
NVP-based regimen initiated before pregnancy 2 3.5 56 9.28 2.27–37.94 0.002
Sensitivity Analysis (including initiation during first 15 weeks post LMP)
ART initiated before LMP + during T1 6 0.6 998 1.56 0.65–3.75 0.319
EFV-based regimen initiated before LMP + during T1 3 0.3 915 0.85 0.26–2.79 0.791
NVP-based regimen initiated before LMP + during T1 2 3.2 61 8.52 2.08–34.90 0.003
NVP, Nevirapine; LMP, last menstrual period; CI, confidence interval.
Risk Analysis B, comparing the risk of the composite endpoint of other ABOs with ART initiation (1) at any time during pregnancy, (2) before and (3) during pregnancy is reflected in Table 6.
TABLE 6: Risk of other adverse birth outcomes:† Analysis B.
Risk analyses Number of ABOs N Unadjusted risk ratio p Adjusted risk ratio p
N % Risk ratio 95% CI Risk ratio 95% CI
Singleton births to HIV negative women 1597 26.0 6134 1.00
ART initiated any time during or before pregnancy 1069 29.9 3577 1.15 1.08–1.23 < 0.001 1.23 1.14–1.31 < 0.001
ART initiated before conception 118 31.2 378 1.20 1.03–1.40 0.022 1.34 1.14–1.58 0.001
ART initiated during pregnancy 617 29.2 2112 1.12 1.04–1.21 0.004 1.20 1.11–1.30 < 0.001
EFV-based regimen initiated any time during or before pregnancy 1015 29.8 3411 1.14 1.07–1.22 < 0.001 1.22 1.14–1.31 < 0.001
EFV-based regimen initiated before conception 93 30.6 304 1.18 0.99–1.40 0.07 1.31 1.09–1.57 0.004
EFV-based regimen initiated during pregnancy 608 29.2 2083 1.12 1.04–1.21 0.005 1.20 1.11–1.30 < 0.001
NVP-based regimen initiated any time during or before pregnancy 32 33.3 96 1.28 0.96–1.70 0.09 1.46 1.10–1.95 0.01
NVP-based regimen initiated before conception 20 34.5 58 1.32 0.93–1.89 0.123 1.54 1.07–2.20 0.019
NVP-based regimen initiated during pregnancy 3 30.0 10 1.15 0.45–2.97 0.769 1.29 0.50–3.32 0.6
ART initiated before conception 118 31.2 378 1.00 - - - - -
ART initiated during pregnancy 617 29.2 2112 0.94 0.79–1.10 0.427 0.90 0.76–1.07 0.237
EFV-based regimen initiated before conception 93 30.6 304 1.00 - - - - -
EFV-based regimen initiated during pregnancy 608 29.2 2083 0.95 0.80–1.14 0.613 0.93 0.77–1.12 0.42
NVP-based regimen initiated before conception 20 34.5 58 1.00 - - - - -
NVP-based regimen initiated during pregnancy 3 30.0 10 0.87 0.32–2.39 0.787 0.57 0.17–1.98 0.379
EFV-based regimen initiated before conception 93 30.6 304 1.00 - - - - -
NVP-based regimen initiated before conception 20 34.5 58 0.89 0.60–1.31 0.551 0.80 0.54–1.19 0.267
ABOs, adverse birth outcomes; ART, antiretroviral treatment; EFV, Efavirenz; NVP, Nevirapine; CI, confidence interval.
†, Other ABOs include pregnancy losses, early neonatal deaths, preterm delivery and small for gestational age.
http://www.sajhivmed.org.za 362 Open Access