Page 12 - Nursing Matters June 2021 Vol 12
P. 12
ITREMA’s second key message of uncertainty about adherence to ART. in clinical practice.
suggests that response to viral Patients in general tend to underreport
rebound is delayed. How are non-adherence. 6,7 Therefore, healthcare Patient adherence to treatment can
patients with viral rebound workers often assume that patients also be examined in the laboratory
followed-up in clinical practice in are non-adherent, give intensified using tests that measure the level of
South Africa? adherence counselling and defer the ARV drugs in patient samples. Such
switch to second-line ART. This may lead tests, referred to as drug level testing,
We found that in South African HIV to resuppression of the VL in some cases. can be performed in a variety of ways.
treatment facilities, patients on first-line ART However, it is unlikely to have an effect in As part of the ITREMA project, novel
with viral rebound did not always receive a patients who have drug resistant HIV. To methods have been evaluated for drug
confirmatory VL within the recommended address this problem, healthcare workers level testing for efavirenz, lopinavir,
timeframe. Those patients who did need to know whether the patient has an and dolutegravir. These methods are
receive a VL confirming virological failure adherence problem and if the patient affordable and relatively easy to
were not always switched to second- has drug resistant HIV. implement in the laboratory. In ITREMA,
line ART. Additionally, the patients that the tests were used as point-of-care tests
were switched to second-line ART were, How can we enable healthcare and turn-around times of 30 minutes
on average, only switched after one workers to assess adherence were achieved. Currently, availability of
8
year following an elevated VL (Fig. 1). 2 and viral drug resistance? these tests in South Africa is still limited to
Patients with an unsuppressed VL are able academic and research settings.
to transmit HIV to others and to develop Resistance of the HIV virus to ART can be
resistance therefore, it is crucial to minimise detected using drug resistance testing, Could these novel drug level
this delay as much as possible. which is available to South African public testing methods be used as
sector healthcare facilities. However, a tool to determine patient
What were the reasons for drug resistance testing is expensive and treatment adherence?
the difference between the is therefore reserved for use in second-
guideline recommendations and line treatment failure. It is of questionable In one of ITREMA’s projects, efavirenz,
the observed real-life situation? value in first-line treatment failures. New lopinavir, and dolutegravir drug level
and less expensive methods for drug testing was implemented as a qualitative
Healthcare workers often reported not resistance testing are in development but test. The test result can inform the clinician
switching to second-line ART that because unfortunately are not currently available whether there is a detectable drug level
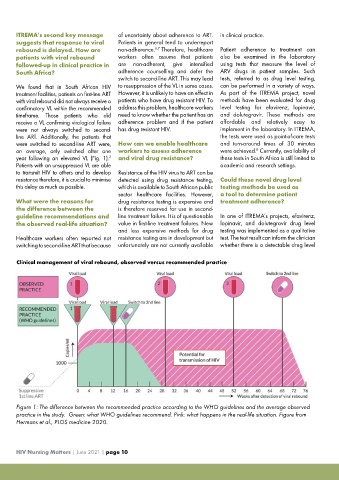
Clinical management of viral rebound, observed versus recommended practice
Figure 1: The difference between the recommended practice according to the WHO guidelines and the average observed
practice in the study. Green: what WHO guidelines recommend. Pink: what happens in the real-life situation. Figure from
Hermans et al., PLOS medicine 2020.
HIV Nursing Matters | June 2021 | page 10